TOCOLYSIS in 2016: An Evidence-Based Clinical Review
1Ann-Sophie Page, 2Geert Herman Page 1,University Hospital Ghent, Belgium
2Jan Yperman Hospital Ypres, Belgium
Email: 1annsophie.page@gmail.com , 2geert.page@skynet.Be
Abstract
To gather and appraise the most recent evidence about the effectiveness of tocolysis to prevent bad perinatal outcomes in preterm births. A systematic literature review was carried out up to April 2016 along with the analysis of data presented during several international congresses. The diagnosis of preterm labour is now made reasonably accurately using cervical length measurement, combined with a (variant of the) fibronectin test. Tocolysis may, in comparison with placebo (53%) delay birth by 48 hours (75-93%). The added value of transportation to a tertiary centre with a neonatal intensive care unit (NICU) is no longer in question, and neither is the added value of antenatal corticosteroids. Two tocolytic agents, nifedipine and atosiban, are currently used for this purpose. However, there is still a demand for randomised evidence of the effect of tocolysis on better perinatal outcomes. Tocolysis for 48 hours is recommended to allow for intra-uterine transport and the timely delivery of antenatal corticosteroids. Future research has to focus on efficient and safe tocolytic agents. Atosiban is currently the product with the best "effectiveness/side-effect ratio" to postpone delivery in preterm labour, when indicated.
Keywords
Tocolysis, preterm, labour, perinatal outcome
I. Introduction
Premature neonates are directly (35%) and indirectly (15%) responsible for 50% of neonatal mortality [1]. The main indirect causes of perinatal mortality after premature birth are sepsis, pneumonia and intra-partum asphyxia. Research has shown that gestation (< 34 weeks) has a greater influence on neonatal outcomes than birth weight. Table 1 shows the importance of gestational age and the limits within which tocolysis can be used. These limits are subjective but some relevant values do exist. After 34 weeks, the benefit from delaying the birth appears not to be very meaningful in regard to incidence of grade III and IV intraventricular hemorrhage, necrotizing enterocolitis, and sepsis [3]. Stratification by gestational age is therefore important in analysing outcomes. Table 1 shows the neonatal morbidity and mortality based on gestational age.
Recent insights into the pathophysiology of premature birth [4] have shown that there are many causes of preterm birth (Figure 1). Premature birth should be seen more as a syndrome, and most spontaneous births after 34 weeks should be seen as physiological rather than pathological (chronic chorioamnionitis). On the other hand, preterm labour due to reduced placental blood flow has more influence on preterm birth at 28 to 33 weeks' gestation. In this case administration of corticosteroids [5] and possibly MgSO4 is very useful.
The diagnosis of preterm labour is now made reasonably accurately using cervical length measurement, combined with a (variant of the) fibronectin test (Figure 2). Combining these two tests offers a sensitivity of 80 to 86% and a specificity of 61 to 100% in predicting imminent preterm birth [2].
Tocolysis may, in comparison with placebo (53%) delay birth by 48 hours (75-93%) [6]. There is no doubt that tocolysis has its place, certainly where the membranes are intact. All that remains is to choose between the agents currently available. Currently all guidelines throughout the world only consider nifedipine and atosiban (with a few exceptions for COX inhibitors). What clinicians are now mainly asking is, which of these tocolytics should be used to delay the premature birth by 48 hours. The evidence is clear that this period of time is sufficient to create the opportunity for intrauterine transport and administration of corticosteroids with a maximal respiratory effect. These therapies have proven their efficacy in optimising neonatal outcomes [5]. Viewing perinatal outcomes, as an endpoint for the effect of tocolysis is not, however, quite so useful, since its efficacy is also partly determined by neonatal care and very large trials would be required to reach a statistically significant conclusion. Logically the product with the greatest efficacy and the fewest side effects should be preferred.
This review sets out the latest reliable evidence in this area.
II. Patients and Methods
A systematic literature review was carried out up to April 2016 along with the analysis of data presented during the Obstetrics Forum Congress in Prague [7], the DIP Congress in Berlin [8], the FMF World Congress in Crete [9] and the Congress on Perinatal Medicine in Maastricht [10].
III. Results and Discussion
Considerable progress has been made in primary prevention (interventions directed to all women implemented before or during pregnancy, to reduce risks). Prediction models as cervical length measurement during first trimester scan are examples of primary prevention) and secondary prevention (measures directed toward reducing the risk in women with already assessed risk factors) of preterm labour and birth. This could make it possible to reduce the number of preterm births in the near future by 30 or possibly 40% [7]. Many predictive models allow a high-risk group of people to be defined who are eligible for secondary prevention [7]. Secondary prevention may consist of bed rest, administration of vaginal progesterone, application of a cerclage or insertion of a cervical pessary [2]. Tertiary prevention consists of tocolysis using active tocolytics such as nifedipine or atosiban. This review focuses on tertiary prevention.
Tocolysis is primarily intended to achieve a prolongation of the pregnancy for about two days in order to provide effective treatment with corticosteroids to optimise fetal lung maturation, not to extend the duration of the pregnancy itself. Administration of magnesium sulphate, if indicated, and referral to tertiary care may also take place during these important 48 hours. The indications for tocolysis are accepted worldwide and presented in a flow chart (Figure 2) [2]. The contra-indications for tocolysis are maternal heart disease, renal disease or infection, intrauterine infection, severe vaginal bleeding, severe preeclampsia placental detachment or suspected fetal distress, intrauterine death, fetal anomalies incompatible with extra- uterine life and very severe fetal growth restriction. Ruptured membranes are currently under debate [10,11]. Tocolysis in this group causes more chorioamnionitis, low Apgar scores and a greater requirement for ventilation and should therefore be strongly questioned.
As a guideline for defining the dose, the treatment regimes set out in the APOSTEL III study protocol can be used. Nifedipine is started at 2 x 10 mg nifedipine capsules taken orally in the first hour, followed by 20 mg nifedipine retard every 6 hours for the next 47 hours. In the atosiban group, a bolus injection of 6.75 mg i.v. is given over 1 minute, followed by 18 mg/hour for 3 hours, followed by a maintenance dose of 6 mg/hour for 45 hours.
The added value of transportation to a tertiary centre with a neonatal intensive care unit (NICU) is no longer in question, and neither is the added value of antenatal corticosteroids. In Cochrane meta-analysis [5] the data showed that at 23 weeks +0 days' gestation to 33 weeks +6 days' gestation, treatment with corticosteroids was associated with a risk reduction (RR) of 0.66 (95% CI:0.59-0.73) or a NNT (number needed to treat) of 11 (9-14) for respiratory distress syndrome (RDS). This study also showed a risk reduction RR of 0.69 (95% CI: 0.58-0.81) and an NNT of 22 (16-36) to avoid neonatal mortality (Table 2). The respiratory effect is seen less than 24 hours after the first dose [5]. A favourable effect is also observed on the incidence of intra- ventricular haemorrhage (IVH) with a RR= 0.54 (95% CI:0.43-0.69), necrotising enterocolitis (NEC) with a RR=0.46 (95% CI:0.29-0.74) and admission to a neonatal care ward (RR=.0.80 (95% CI: 0.65-0.99)). Currently only a single rescue dose of corticosteroids is considered in the case of a newly occurring risk at less than 32 weeks' gestation and if more than 14 days have passed since the first dose was administered [10] . The long-term effects of antenatal corticosteroids, certainly when administered repeatedly, are still unknown and are increasingly controversial. Higher rate of cerebral palsy among children who had been exposed to repeat doses of corticosteroids is of concern although the difference was not statistically significant as mentioned in one of the research study [12]. In women with threatened preterm birth, delay of delivery by 48 h allows antenatal corticosteroids to improve neonatal outcomes [13].
Magnesium sulphate (MgSO4) is recommended for imminent birth (<12 hours) at less than 32 weeks. Magnesium sulfate administered to women at risk of delivery before 34 weeks of gestation reduces the risk of cerebral palsy [14]. Due to the intrinsic action of MgSO4 and the timing of administration of this important treatment to prevent cerebral palsy (CP), RR=0.69 (95% CI:0. 55-0.88), this intervention and its outcomes do not contribute much to perinatal outcomes (no overall difference in the risk of total pediatric mortality) in the context of tocolytic treatment [14]. Considering starting MgSO4 for neuroprotection presupposes stopping tocolysis if this has been started [10]. The recommended dose is a loading dose of 6 g MgSO4 IV over a period of 20-30 minutes, followed by a maintenance dose of 2 grams per hour until birth or for 12 to a maximum of 24 hours [10].
The current guidelines for tocolysis, WHO [15], RCOG-NICE [16], ACOG [17] and KCE [2] still include two products for carrying out tocolysis, namely nifedipine and atosiban. On what evidence is this choice based?
Other products such as COX -inhibitors, MgSO4 and beta agonists are no longer indicated for tocolysis due to lower efficacy and/or more side effects [2, 15, 16].
The Haas DM et al., [18] in 2012 designed a systemic review and network meta-analysis in which investigators identified 95 randomized, controlled trials of tocolytic therapy. This analysis showed that the probability of delivery being delayed by 48 hours was greatest with prostaglandin inhibitors (odds ratio [OR], 5.39; 95% confidence interval [CI], 2.14–12.34), followed by magnesium sulfate (OR, 2.76; 95% CI, 1.58–4.94), calcium channel blockers (OR, 2.71; 95% CI, 1.17– 5.91), beta-mimetics (OR, 2.41; 95% CI, 1.27– 4.55), and the oxytocin receptor blocker atosiban (OR, 2.02; 95% CI, 1.10–3.80), compared with placebo. Also it indicated, no class of tocolytic was superior to placebo in reducing the incidence of neonatal respiratory distress syndrome. Side effects that required a change of medication were significantly more common with beta mimetics (OR, 22.68; 95% CI, 7.51–73.67), magnesium sulfate (OR, 8.15; 95% CI, 2.47–27.70), and calcium channel blockers (OR, 3.80; 95% CI, 1.02–16.92), compared with placebo [18]. The authors concluded, prostaglandin inhibitors and calcium channel blockers were the tocolytics with the best probability of being ranked in the top three medication classes for the outcomes of 48 hour delay in delivery, respiratory distress syndrome, neonatal mortality, and maternal side effects (all cause) [18].
There were hopes that the APOSTEL III trial, a multicenter, randomized controlled trial, that has just been published could offer more clarity concerning the choice that has to be made between the two tocolytics i.e., nifedipine and atosiban [13]. The authors of the APOSTEL III trial, however, concluded that both products, if used for 48 hours, offer equal perinatal outcomes and that large, placebo-controlled trials with sufficient power to analyse serious perinatal outcomes should provide an answer to the question of which product to choose. An analysis of this APOSTEL III trial does in fact teach us that the two products are equally efficacious for delaying birth by 48 hours and result in similar adverse perinatal outcome rate. (The primary outcome was a composite of adverse perinatal outcomes, which included perinatal mortality, bronchopulmonary dysplasia, sepsis, intraventricular haemorrhage, periventricular leukomalacia, and necrotising enterocolitis). Although delaying birth by 48 hours was a secondary outcome measure in the study, it was still large enough to reach this conclusion. It should be noted, however, that it involved women with threatened preterm birth (gestational age 25–34 weeks) who were randomly assigned (1:1) to either oral nifedipine or intravenous atosiban for 48 h. This study [13] indicated, primary composite outcome measure of perinatal mortality and diverse perinatal morbidities showed no significant difference between the two products as primary outcome occurred in 14% in the nifedipine group and in 15% in the atosiban group (RR 0•91, 95% CI 0•61–1•37) and, 5% babies died in the nifedipine group whereas 2% died in the atosiban group (RR 2•20, 95% CI 0•91–5•33); all deaths were deemed unlikely to be related to the study drug. The authors rightly pointed out that this could still be attributed to chance (the study did not have sufficient power to make any statement about this), but it still calls the safety of nifedipine into question. Maternal adverse events did not differ between groups [13].
No pronounced side effects of atosiban have been demonstrated in the literature and the product is registered for the indication of tocolysis. Nifedipine, on the other hand, is an antihypertensive, which is not registered for this indication (off-label use), so there are no robust methods to test the safety of the product apart from the ‘product-license’ procedure.
There is also evidence that nifedipine, by lowering maternal blood pressure, can cause reduced placental perfusion, with cases described of neonatal death and serious morbidity. Maternal hypotension and tachycardia resulting from nifedipine use have been demonstrated in the observational literature [19]. Finally, we realise that the pregnant population is changing. Women are becoming pregnant later in life and obesity, hypertension and cardiovascular disorders are on the increase. Observational research has shown that rise in maternal mortality can be attributed to cardiovascular disorders, a trend which is continuing in view of the increasing comorbidity in pregnant women [20].
Meanwhile the APOSTEL IV study (nifedipine in PPROM) has been stopped prematurely [21], probably due to new external evidence concerning nifedipine. The new European Guidelines currently also present atosiban as the first-choice tocolytic [10].
Table 1: Neonatal morbidity and mortality based on gestational age [3].
| Gestation, weeks | Survival | Respiratory distress syndrome | Intraventricular bleeding | Sepsis | Necrotising enterocolitis | No morbidity |
| 24 | 40% | 70% | 25% | 25% | 8% | 5% |
| 25 | 70% | 90% | 30% |
29% |
17% |
50% |
| 26 | 75% |
93% |
30% | 30% | 11% | 60% |
| 27 | 80% | 84% | 16% | 36% | 10% | 70% |
| 28 | 90% | 65% | 4% | 25% | 25% | 80% |
| 29 | 92% | 53% | 3% | 25% | 14% | 85% |
| 30 | 93% | 55% | 2% | 11% | 15% | 90% |
| 31 | 94% | 37% | 2% | 14% | 8% | 93% |
| 32 | 95% | 28% | 1% | 3% | 6% | 95% |
| 33 | 96% | 34% | 0% | 5% | 2% | 96% |
| 34 | 97% | 14% | 0% | 4% | 3% | 97% |
Table 2: Results of using antenatal corticosteroids (versus placebo or no treatment) on the risk of development of RDS and neonatal mortality (21 studies involving 3885 women and 4269 babies). RDS: respiratory distress syndrome. RR: relative risk; NNT: numbers needed to treat [5].
| Antenatal corticosteroids | RR (confidence interval) | NNT (confidence interval) |
| RDS | 0.66 (0.59-0.73) | 11 (9-14) |
| NEONATAL MORTALITY | 0.69 (0.58-0.81) |
22 (16-36) |
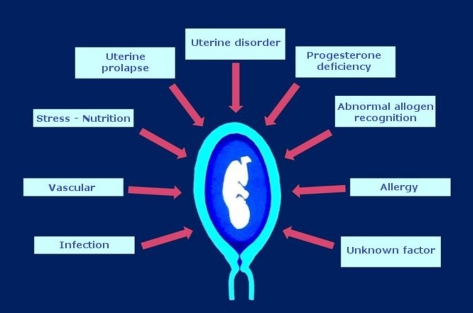
Figure 1: Main causes of preterm birth [4]
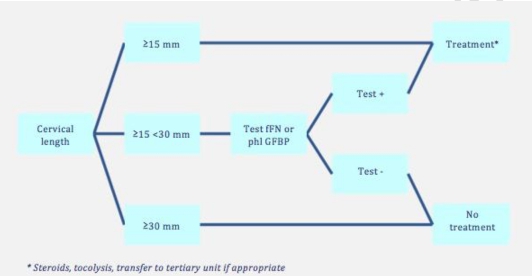
Figure 2: Algorithm for preterm birth (taken from [2]) fFN: fetal fibronectin test, phlGFBP: phosphorylated insulin- like growth factor binding protein.
IV. Conclusion
The current evidence concerning the impact of tocolysis on important perinatal outcomes remains unclear. It has been shown that tocolysis can delay birth by 48 hours and this is probably its only purpose; to allow corticosteroids to be administered antenatally and to provide intrauterine transport if appropriate. The clinician therefore only has to make a choice between nifedipine and atosiban, which both appear to be equally effective. The latter, however, demonstrates a clearly superior safety profile, which, in view of the constantly increasing co-morbidity in the pregnant population, is certainly becoming more important. Moreover, atosiban is licensed for this indication. Despite the additional cost, which can be limited through careful diagnosis of true preterm labour, respecting the period of gestation during which tocolysis is helpful and limiting administration to 48 hours, atosiban is currently the product with the best "effectiveness/side-effect ratio". Since the very recent European Guidelines also concur with this, the choice for the clinician appears to be obvious.
It may also be appropriate to carry out double- blind, randomised studies with sufficient ‘power’ to compare the two products and demonstrate a significant difference between the two in delaying birth by 48 hours. At the same time the safety aspects of both products must be followed up. Future clinical research should focus on large placebo-controlled trials, powered for perinatal outcomes.
Consequently, while awaiting possible new tocolytics, atosiban is the first-choice tocolytic, to be administered for a maximum of 48 hours, for the correct indications and at the correct dose, to women at serious risk of pre- term birth.
Aknowledgments
Author’s contribution to the manuscript: AS Page: data collection, data analysis GH Page: manuscript writing
Conflict of interest:
Ann-Sophie Page declares that she has no conflict of interest.
GH Page has received financial support for attending symposia by Ferring N.V.
Compliance with Ethical Standards
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
V. References
[1].Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al (2013) Born too soon: the global epidemiology of 15 million preterm births. Reprod Health,10,1:S2.
[2]. KCE Reports 228A (2014) Preventie bij verhoogd risico op vroeggeboorte - evaluatie van een aantal courante interventies. Federaal Kenniscentrum voor de gezondheidszorg: Good Clinical Practice.
[3].Robertson PA,Sniderman SH,Creasy RK.(1992) Neonatal morbidity according to gestational age and birth weight from five tertiary care centres in the USA,1983 through 1986. Am J Obstet Gynecol,166(6):1629-1645.
[4].Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM.(1994) The preterm labor syndrome. Ann N Y Acad Sci,734:414- 29.
[5]. Roberts D, Dalziel S. (2006) Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 3:CD004454.
[6]. Haas DM, Imperiale TF, Kirckpatrick PR,Klein RW,Zollinger TW,Golichowski AM. (2009) Tocolytic Therapy: A meta-analysis and decision analysis. Obstet Gynecol, 113:585-94.
[7]. Obstetrics Forum Congres11-13 Mach 2015; Prague.
[8]. The 8th International DIP symposium. Diabetes, hypertension, metabolic syndrome & pregnancy.15-18 April 2015; Berlijn.
[9].14th World Congress in Fetal Medicine (2015) Crete.
https://fetalmedicine.org/abstracts/2015/html/preter m-birth.html
[10]. XXV European Congress Perinatal Medicine, Maastricht (2016). The Journal of Maternal-Fetal & Neonatal Medicine, 29:298.
[11]. Mackeen AD, Seibel-Seamon J, Muhammad J, Baxter JK, Berghella V. (2014) Tocolytics for preterm premature rupture of membranes. Cochrane Database Syst Re, 2:CD007062.
[12]. Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, et al. (2007) Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med, 357(12):1190-8.
[13]. van Vliet EO, Nijman TA, Schuit E, Heida KY, Opmeer BC, Kok M, et al. (2016) Nifedipine versus atosiban for threatened preterm birth (APOSTEL III): a multicentre, randomised controlled trial. Lancet, 387, 2117- 2123.
[14]. Conde-Agudelo A, Romero R. (2009) Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks' gestation: a systematic review and metaanalysis. Am J Obstet Gynecol, 200(6):595-609.
[15]. World Health Organization. WHO recommendations on intervention to improve preterm birth outcomes. (2015) Genev. http://www.who.int/reproductivehealth/publica tions/preterm-birth-guideline
[16]. Royal College of obstetricians and gynecologists (2002):clinical green top guidelines.Tocolytic drugs for women in prterm labour. http://www.rcog.org.uk/guidelines/tocolytics.ht lm
[17]. ACOG Practice Bulletin no. 159: Management of preterm labor (2016) Obstet Gynecol, 127(1): e29-e38.
[18]. Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. (2012) Tocolytic therapy for preterm delivery: systematic review and network meta-analysis. BMJ, 345:e6226.
[19]. Van Geijn HP, Lenglet JE, Bolte AC. (2005) Nifedipine trials: effectiveness and safety aspects. BJOG, 112: 79-83.
[20]. Schutte JM, Steegers EA, Schuitemaker NW, Santema JG, de Boer K, Pel M, et al. (2010) Rise in maternal mortality in the Netherlands. BJOG, 117(4):399-406.
[21]. Nijman T, van Vliet E, Naaktgeboren C, et al. (2016) Nifedipine versus placebo in the treatment of preterm prelabour rupture of membranes: a randomized controlled trial: Assessment of perinatal outcome by use of tocolysis in early labor – APOSTEL IV trial. European Journal of Obstetrics & Gynecology and Reproductive biology, 205:79-84.
