The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine abstinent mice
Authors: Panos Zanos (PhD)*, Polymnia Georgiou (BSc)*, Sherie R. Wright (PhD), Susanna M. Hourani (PhD), Ian Kitchen (PhD), Raphaëlle Winsky-Sommerer (PhD)#, Alexis Bailey (PhD)#
Abstract
The main challenge in treating opioid addicts is to maintain abstinence due to the affective consequences associated with withdrawal which may trigger relapse. Emerging evidence suggests a role of the neurohypophysial peptide oxytocin in the modulation of mood disorders as well as drug addiction. However, its involvement in the emotional consequences of drug abstinence remains unclear. We investigated the effect of 7-day opioid abstinence on the oxytocinergic system and assessed the effect of the oxytocin analogue carbetocin on the emotional consequences of opioid abstinence, as well as relapse. Male C57BL6J mice were treated with a chronic escalating-dose morphine regimen (20-100 mg/kg/day, i.p.). Seven days withdrawal from this administration paradigm induced a decrease of hypothalamic oxytocin levels and a concomitant increase of oxytocin receptor binding in the lateral septum and amygdala. While no physical withdrawal symptoms or alterations in plasma corticosterone levels were observed after seven days of abstinence, mice exhibited increased anxiety-like and depressive-like behaviors and impaired sociability. Carbetocin (6.4 mg/kg, i.p.) attenuated the observed negative emotional consequences of opioid withdrawal. Furthermore, in the conditioned place preference paradigm with 10 mg/kg morphine conditioning, carbetocin (6.4 mg/kg, i.p.) was able to prevent the stress-induced reinstatement to morphine-seeking following extinction. Overall, our results suggest that alterations of the oxytocinergic system contribute to the mechanisms underlying anxiety, depression and social deficits observed during opioid abstinence. This study also highlights the oxytocinergic system as a target for developing pharmacotherapy for the treatment of emotional impairment associated with abstinence and thereby prevention of relapse.
Keywords: opioid, oxytocin, abstinence, relapse, depression, sociability
Introduction
Although some neurotransmitter systems (e.g., serotonin and corticotropin-releasing factor) have been suggested to be involved (Goeldner et al, 2011; Koob and Kreek, 2007; Koob, 2008), the mechanisms underlying the emotional impairment associated with opioid abstinence remain largely unclear. Several lines of evidence suggest a role of the “social” peptide oxytocin (OT) in drug addiction. OT-producing neurons located in the hypothalamus innervate brain regions associated with drug-seeking behavior as well as stress, mood, fear and emotionality, such as the amygdala, septum and the bed nucleus of stria terminalis, where oxytocin receptors (OTRs) are expressed (Gimpl and Fahrenholz, 2001). OT is involved in the regulation of stress via its action on the hypothalamo-pituitary-adrenal (HPA) axis (Windle et al, 2004), and has been also implicated in the modulation of emotional and social behaviors (Neumann and Landgraf, 2012). In particular, OT promotes social bonding as well as social memory (Keverne and Curley, 2004) and exerts a potent anxiolytic, anti-aggressive and antidepressant effect in humans (Heinrichs and Domes, 2008; Liu et al, 2012), and in animal models (Neumann et al, 2012).
There is emerging evidence supporting the involvement of OT in the effects of a number of drugs of abuse and in preventing relapse to drug-seeking (Broadbear et al, 2011; McGregor and Bowen, 2012; McGregor et al, 2008; Sarnyai, 2011). With respect to opioids, an inhibitory effect of OT has been demonstrated on morphine tolerance, physical symptoms of naloxone-precipitated morphine withdrawal (Kovacs et al, 1985; Kovacs et al, 1984) and on heroin self-administration (Kovacs and Van Ree, 1985) in rodents. In addition, marked alterations in OT peptide content and/or synthesis were reported in the forebrain, hippocampus, amygdala and hypothalamus of rodents following acute and chronic opioid administration (Kovacs et al, 1987; You et al, 2000). With respect to relapse, OT was shown to facilitate extinction of methamphetamine-induced conditioned place preference (CPP) and to attenuate stress- (Qi et al, 2009) and priming- (Carson et al, 2010) induced reinstatement of methamphetamine-seeking behavior in rodents. Overall, while the contribution of OT in different stages of drug addiction is recognized, its specific role in the emotional impairment associated with abstinence, which may trigger relapse to drug-seeking, remains largely unknown.
Our aim was to assess whether OT alterations may underlie negative emotional states associated with opioid abstinence. Specifically, we hypothesize that 7-day withdrawal from chronic morphine administration, causes alterations of the central OT-ergic system and that an OT analogue may attenuate the emotional consequences of 7-day opioid abstinence and prevent relapse. We used a mouse model of 7-day, morphine abstinence to study the effects of withdrawal on OT peptide levels and OTR binding in the brain. The emergence of emotional impairment (i.e., anxiety, depressive-like behaviors and sociability deficits) was also assessed following this 7-day withdrawal period. We then examined the effect of the OT analogue carbetocin (CBT) on the negative emotional consequences of withdrawal, as well as the stress-induced reinstatement of morphine-seeking behavior.
Materials and Methods
Animals and paradigm for morphine administration and withdrawal
Male C57BL/6J mice (seven-week old, 20-25 g, Charles River Laboratories, Kingston, UK), were housed individually in a temperature-controlled environment with a 12:12-hour light/dark cycle (lights on: 06:00). Food and water were available ad libitum. Mice were left to acclimatize in their new environment for seven days prior to the experiments and were handled daily. All procedures received a favorable opinion by the University of Surrey Ethics Committee and were approved by the UK Home Office (Animals Act 1986). Mice were randomly assigned to two treatment groups: control saline-treated groups (n=148 in total) and chronic morphine-treated groups (n=161 in total). Mice were injected (i.p.) either with saline (4 ml/kg) or morphine (Sigma-Aldrich, Poole, UK) with a chronic morphine escalating-dose administration paradigm (20 mg/kg on day 1, 40 mg/kg on days 2-3, 80 mg/kg on days 4-5 and 100 mg/kg on days 6-7), twice per day at 09:00 and 17:00, as previously described (Goeldner et al, 2011; Muller and Unterwald, 2004; Zhou et al, 2006), with minor modifications (Fig. S1A). To induce withdrawal, mice were then left in their home cage for 7 consecutive days without receiving any injection, which was previously shown to induce characteristic enhancement of depressive-like behavior in rats (Anraku et al, 2001).
Effect of chronic morphine treatment and 7-day abstinence on the central oxytocinergic system and plasma corticosterone levels
Mice were killed by decapitation either 1 hour after the final treatment injection for the chronic morphine and saline groups, or after 7 days of withdrawal. Brains were frozen in isopentane solution (-20oC) and sectioned for analysis of autoradiographic OTR binding (n=5 per group) as previously described (Zanos et al, 2013). Alternatively, brain regions were dissected (i.e., hypothalamus, septum, amygdala, hippocampus; n=6-7 brains per group) for determining OT peptide levels. Peptide extraction was performed according to Szeto et al, (2011), using a previously described protocol with minor modifications (Christensson- Nylander et al, 1985). Measurement of OT levels was performed on tissue extracts using an Enzyme Immunoassay (Enzo Life Sciences (UK) Ldt, Exeter, UK). Trunk blood was also collected for quantification of plasma corticosterone levels (n=11-12 per group) using a commercially available radioimmunoassay kit, according to manufacturer’s instructions (MP biochemical, New York, NY, USA).
Effect of morphine administration and abstinence on physical symptoms of withdrawal in mice
Basal locomotor activity, number of withdrawal jumps, fecal boli, body weight, food and water intake were recorded daily, at the same time, throughout the chronic morphine administration paradigm and/or at specific time points during the withdrawal period (n=13-18 mice per group; Supplementary Methods).
Effect of morphine abstinence and carbetocin administration on anxiety-, depressive- like and sociability behaviors
A different cohort of mice underwent chronic morphine administration, as described above. Following a 7-day withdrawal period, mice were injected (i.p.) either with saline (4 ml/kg) or CBT (6.4 mg/kg), 15 min prior to measuring anxiety- and depressive-like behavior using the elevated plus-maze and forced-swim tests, or 5 min prior to the assessment of sociability and social novelty behavior using the Crawley’s three-chambered social approach test (for details, see Supplementary Methods). Each behavioral test was performed on a separate group of animals (n=7-10 per group). The dose was chosen based on previous studies (Chaviaras et al, 2010) and a pilot experiment assessing the effects of CBT (i.p.; Vehicle n=6; 2 mg/kg, n=3; 6.4 mg/kg, n=10, and 20 mg/kg, n=3) on depressive-like behavior in mice withdrawn for 7 days from chronic saline or morphine administration, using the forced-swim test (Supplementary Methods and Fig. S2). While CBT did not induce any alterations of parameters characteristic of depressive-like behavior in the saline-withdrawn group, this compound dose-dependently reduced the depressive-like symptoms observed in the morphine-withdrawn mice. We selected the lowest dose able to reverse depressive-like behavior in morphine-abstinent mice, i.e., 6.4 mg/kg. This dose did not have any significant effect on locomotor activity in naïve mice (n=6 per group; data not shown). CBT was selected because of its half-life (85-100 min) compared to OT (3-5 min) and based on its selectivity to the OTR in the rat (Engstrom et al, 1998).
Effect of carbetocin administration on stress-induced reinstatement of morphine- seeking behavior
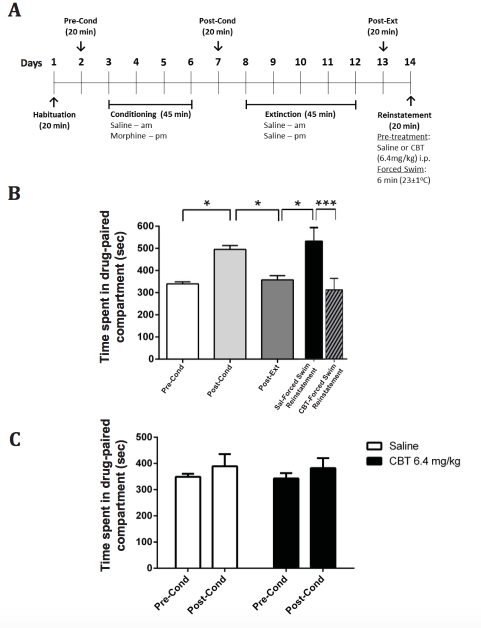
We used a CPP apparatus (Opto-Max Activity Meter v2.16, Columbus Instruments, OH, USA), as previously described (Bailey et al, 2010). The CPP reinstatement protocol was modified from (Mantsch et al, 2010). Briefly, it consisted of a habituation session, pre- conditioning test, 4 conditioning sessions (morning saline and 4 hours later a 10 mg/kg morphine subcutaneous (s.c.) injections daily), a post-conditioning test, 5 extinction sessions (i.e., morphine injection was replaced by a saline injection), a post-extinction test and a reinstatement session, each carried out on consecutive days (Fig. 5A). During the reinstatement session, mice were pre-treated with either saline (4 ml/kg, i.p.; n=14 mice) or CBT (6.4 mg/kg, i.p.; n=12 mice) and after 5 minutes, they were exposed to a 6-min forced- swim session. Following the forced-swim stress, mice were towel-dried and placed in the CPP apparatus for 20 minutes. Time spent in each compartment was measured during the last 15 minutes of the session. Mice were killed 30 minutes after the reinstatement session and trunk blood was collected for quantification of plasma corticosterone levels.
To ascertain that CBT does not have any rewarding or aversive effects on its own when administered chronically, the effects of CBT (6.4 mg/kg, i.p.) were compared to saline in a CPP paradigm, consisting of one habituation, one pre-conditioning, four conditioning (with saline injection in the morning and CBT administration 4 hours later) phases and one post- conditioning phase. Time spent in each compartment was assessed during the last 15 minutes of the 20-min post-conditioning session and compared in the CBT- and saline-treated groups (n=6 per group).
Statistical Analyses
All the values were expressed as mean ± SEM. For analyses of OTR binding, OT peptide and corticosterone levels, two-way ANOVA with factors ‘treatment’ (i.e., saline or morphine) and ‘7-day withdrawal’ was performed. The effects of CBT on the elevated plus-maze, forced-swim test and sociability tests were compared by two-way ANOVA with factors ‘treatment’ (i.e., saline or morphine) and ‘CBT’ (i.e., CBT or saline). Differences in stress- induced reinstatement of morphine-seeking were analyzed using two-way ANOVA with factor ‘CPP phase’ (pre-conditioning, post-conditioning, post-extinction, reinstatement) and ‘experiment’ (i.e., CBT or saline). The effects of CBT on CPP and locomotor activity were assessed by repeated measures two-way ANOVA with factors ‘CPP phase’ and ‘CBT’ (i.e., CBT or saline), and factors ‘time’ and ‘CBT’ (i.e., CBT or saline) respectively. Differences in weight changes, food and water intake were assessed by two-way repeated measures ANOVA with factors ‘treatment’ (i.e., saline or morphine) and ‘days’. Basal locomotor activity was analysed by repeated measures two-way ANOVA with factors ‘treatment’ (i.e., saline and morphine) and ‘days’. ANOVAs were followed by a Holm-Sidak post-hoc test when significance was reached (i.e. p<0.05). All relevant F-values are provided in Table 1. All statistical analyses were performed using SigmaPlot (Systat Software Inc, London, UK).
Results
Effect of chronic morphine administration and 7-day withdrawal on the central oxytocinergic system and plasma corticosterone levels
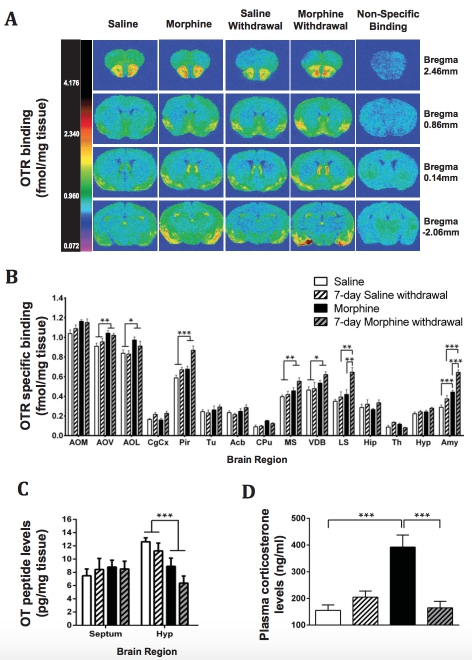
We first investigated the effects of chronic, escalating-dose morphine administration and 7- day withdrawal on OTR binding in the brain (Fig. 1A-B; Table 1). A significant effect of the 7-day morphine treatment was observed in several brain regions (Table 1). The morphine- treated groups showed an increase in OTR binding in the piriform cortex, medial septum, vertical limb of the diagonal band of Broca, as well as the anterior olfactory nucleus-ventral and -lateral, compared to the control saline-treated groups (Table 1). In the lateral septum and amygdala, a significant ‘treatment’ x ‘withdrawal’ interaction was identified (Table 1). While chronic morphine administration did not alter the OTR binding in the lateral septum, we observed a significant increase in the 7-day morphine-withdrawn group compared to the saline-withdrawn group and to the chronic morphine-treated group (Fig. 1A-B). In the amygdala, the OTR binding was increased both following chronic morphine administration and after 7-day morphine withdrawal compared to control groups. Moreover, the OTR binding showed a significant 1.5-fold increase following the 7-day withdrawal period compared to the morphine administration group (Fig. 1A-B).
We also assessed OT peptide levels in several brain regions. A significant ‘treatment’ effect (Fig. 1C; Table 1) was observed in the hypothalamus, with a significant decrease of OT levels in the morphine-treated groups compared to the control groups, while no changes were observed in the septum. OT content in the hippocampus and amygdala was below the detection level (data not shown).
To assess the effect of chronic morphine treatment and withdrawal on HPA axis activity, we measured plasma corticosterone levels at the end of the chronic administration paradigm and after a 7-day withdrawal period. Chronic morphine treatment increased plasma corticosterone levels, while levels were comparable to the control group following a 7-day morphine withdrawal period (Fig. 1D; Table 1).
Effect of chronic morphine treatment and subsequent abstinence on physical and behavioral withdrawal symptoms
Acute, 1-day withdrawal from chronic morphine treatment induced withdrawal jumping (40 ± 13 vs 0 ± 0 jumps; p<0.001; n=13-18 per group) and increased fecal boli production (12 ± 1 vs 3 ± 1; p<0.001; n=13-18 per group), compared to the saline control group. In contrast, no significant differences between the morphine- and saline-treated groups were observed in either jumping behavior (0 ± 0 vs 0 ± 0 jumps; p>0.05) or defecation (4 ± 1 vs 4 ± 1; p>0.05) following 7-day withdrawal. Chronic morphine administration induced a significant decrease in food and water consumption, associated with a reduction in body mass (Supplementary Fig. 1S; Table 1). The drug withdrawal period was characterized initially by an increase in body mass, as well as food and water intake. Water consumption and body weight normalized to levels similar to saline-treated animals at the end of the 7-day withdrawal period (Supplementary Fig. S1). Moreover, morphine treatment reduced basal horizontal activity from Day 3 to 7 of the administration paradigm (Supplementary Fig. S3A; Table 1). This reduction persisted following the 7-day withdrawal period (Supplementary Fig. S3A). No alteration of basal vertical activity was observed between saline- and morphine-treated mice (Supplementary Fig. S3B).
Effect of carbetocin, an oxytocin analogue, on anxiety-like, depressive-like and sociability behaviors associated with 7-day morphine withdrawal
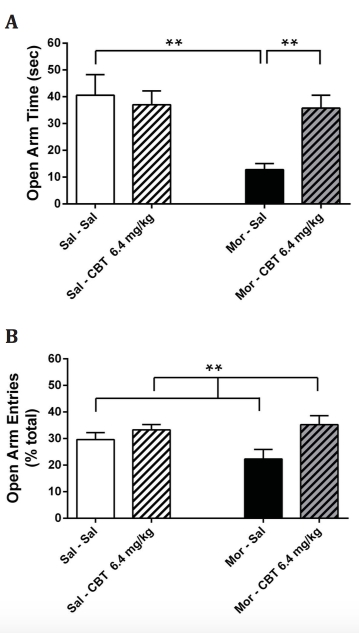
We assessed anxiety-like behavior following the 7-day withdrawal from morphine using the elevated plus-maze. Seven-day withdrawal from morphine decreased the time spent in the open arms and the percentage of open arm entries compared to the control group (Fig. 2A-B), indicating anxiety-like behavior in rodents. Administration of CBT prior to the behavioral test increased the time spent in the open arms in morphine-withdrawn mice to levels similar to the control group (Fig. 2A). In addition, CBT increased the number of entries in the open arms (Fig. 2B). CBT treatment did not induce any changes in the time spent and number of entries in the open arms for the control group (Fig. 2A-B).
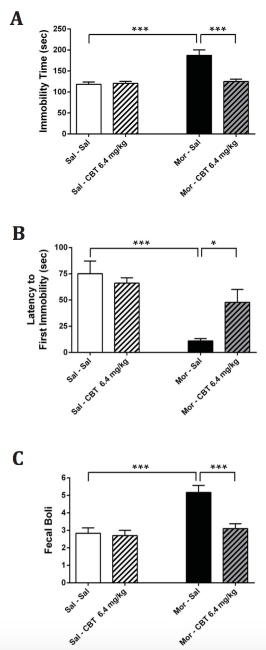
The forced-swim test was used to assess depressive-like behavior following 7-day withdrawal from morphine. Withdrawal increased immobility time (Fig. 3A), and reduced the latency to the first episode of immobility (Fig. 3B). In addition, an increase in fecal boli production was observed (Fig. 3C), also indicative of depressive-like behavior (Craft et al, 2010). CBT reduced the time spent immobile during the forced-swim test, as well as the production of fecal boli, and increased the latency to the first immobility episode, in the morphine- withdrawn group to levels similar to the control group (Fig. 3A-C). Saline-withdrawn mice administered with CBT showed no significant changes in immobility time, latency to first immobility and fecal boli production compared to controls (Fig. 3A-C).
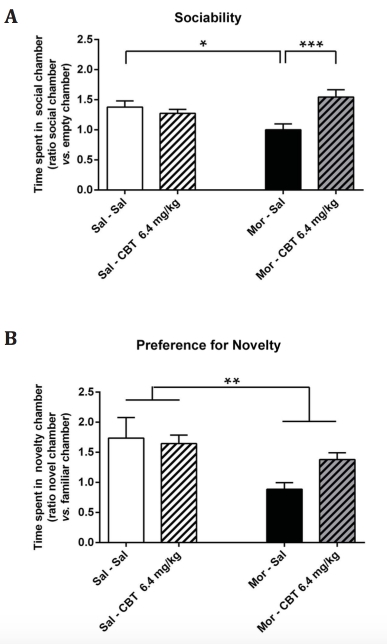
We also assessed mouse sociability using the three-chambered social approach test. Sociability was measured by the time the subject mouse spent in the “social chamber” containing a novel conspecific compared to the “empty chamber”. We found a reduction of sociability behavior in morphine-withdrawn mice compared to the saline-withdrawn group (Fig. 4A). Administration of CBT prior to the behavioral test increased the time spent by morphine-withdrawn mice in the chamber containing the novel mouse compared to the empty chamber (Fig. 4A), indicating a restoration of sociability behavior. CBT had no effect on sociability in control mice undergoing withdrawal from saline (Fig. 4A).
Furthermore, we assessed the preference of the subject mouse for either the chamber with the former conspecific (“familiar chamber”) or the chamber with a second “novel” mouse (“novel chamber”) as indicative of preference for social novelty. Two-way ANOVA revealed an effect of morphine treatment (Fig. 4B) while the interaction ‘treatment’ (morphine vs saline) and ‘CBT’ was close to significance (p=0.06).
Effect of carbetocin on stress-induced reinstatement of morphine-seeking behavior
The CPP reinstatement paradigm is commonly used to assess relapse to drug-seeking (Cordery et al, 2012; Redila and Chavkin, 2008). Morphine administration induced a place preference in the CPP paradigm with an increase in the time spent in the drug-paired compartment in the post-conditioning phase compared to the pre-conditioning phase. The 5- day extinction period led to a significant decrease in the time spent in the drug-paired compartment (post-extinction phase vs post-conditioning phase) (Fig. 5B; Table 1). A forced- swim stress induced reinstatement of the CPP in mice treated with saline, as shown by the increased time spent in the drug-paired compartment compared to the post-extinction phase (Fig. 5B). In contrast, mice pre-injected with CBT did not show this increase in preference for the morphine-paired compartment during the reinstatement phase, compared to the saline pre-treated group (Fig. 5B). Furthermore, no changes in plasma corticosterone levels were observed in the CBT-treated group (244.3 ± 30.0 ng/ml; n=14) compared to the saline control group (252.6 ± 16.7 ng/ml; n=12) following stress-induced reinstatement.
A possible mechanism by which CBT modulates the emotional impairment associated with 7- day morphine withdrawal and the stress-induced reinstatement of morphine CPP is by inducing reward. Thus, we assessed the rewarding or aversive properties of CBT (6.4 mg/kg; i.p.) using the CPP paradigm. CBT did not induce any conditioned place preference or aversion (Fig. 5C; Table 1) compared to the saline control group.
Discussion
This study demonstrated the protective effect of the OT analogue, CBT, on anxiety-, depressive-like behaviors and sociability impairment associated with abstinence from opioid administration, as well as on drug relapse, in a mouse model. In addition, we observed alterations of the OT-ergic system in the brain, which are likely to contribute to the emotional impairment observed following a 7-day withdrawal period from morphine. Altogether these findings highlight a key role of the OT-ergic system in opioid addiction and relapse, suggesting this system as a novel target for the treatment of opioid addiction, and in particular for preventing relapse.
We first established and characterized a mouse model of 7-day opioid abstinence which mimics the physical and emotional symptoms usually displayed by human opioid-withdrawn addicts (Jaffe, 1990; Martin et al, 1969). While acute physical withdrawal symptoms (i.e., withdrawal jumping, defecation, as well as changes in body mass) had disappeared after 7 days of morphine abstinence, anxiety-, depressive-like behaviors were increased and sociability reduced, highlighting the translational value of this model and the direct relationship between opioid abstinence and the emergence of negative emotional state. Consistent with the work of (Zhou et al, 2006), while chronic morphine treatment enhanced the HPA axis activity, corticosterone levels returned to levels similar to those of controls after the 7-day withdrawal period, demonstrating a normalization of the HPA axis. We also demonstrated that chronic morphine administration and/or withdrawal was associated with alterations of the central OT-ergic system. We not only observed reduced levels of OT in the hypothalamus, the main site of OT synthesis in the brain, but also an increase in OTR binding in several regions where OT-producing neurons project (i.e., olfactory nuclei, piriform cortex, septum and amygdala) (Gimpl et al, 2001), suggestive of compensatory mechanisms to the reduced OT-ergic tone. Similar neuroadaptive changes in the oxytocinergic system were previously observed following an acute morphine tolerance paradigm (Sarnyai et al, 1988). Using a specific oxytocin receptor antagonist, Sarnyai et al, showed a key role of oxytocin receptors located in the posterior olfactory nucleus and central nucleus of the amygdala in acute tolerance to morphine. Our findings are also consistent with decreased OT contents in several brain regions following chronic morphine administration in rodents (Kovacs et al, 1987; You et al, 2000). In addition, our data demonstrate that these OT-ergic alterations not only persisted following the 7-day withdrawal period, but was further heightened with respect to OTR binding in the septum and amygdala, regions playing a key role in the regulation of stress and emotions (Phelps and LeDoux, 2005; Singewald et al, 2011). This is in line with a previous study in a rodent model of social deficit in schizophrenia showing that while hypothalamic oxytocin mRNA levels were significantly decreased, oxytocin receptor binding was increased in the central nucleus of the amygdala (Lee et al, 2005). The fact that both the septum and amygdala have been implicated in the stress-regulating and social-enhancing properties of OT (Domes et al, 2007; Lukas et al, 2013) is further suggestive of the OT-ergic system involvement in the negative emotional consequences of opioid withdrawal.
In this translational model, we demonstrated that CBT reversed not only anxiety- and depressive-like behaviors, but also restored sociability in morphine-withdrawn mice to levels similar to the control saline-withdrawn groups. Importantly, CBT did not affect the anxiety level, depressive-like state or sociability in control saline-withdrawn mice. In addition, locomotor activity showed no difference after CBT administration in naïve mice compared to saline-treated mice (data not shown). These findings strongly support the specificity of the anxiolytic, antidepressant and pro-social effects induced by a relatively low dose of CBT to counteract the negative consequences of withdrawal. While CBT shows a high affinity for both OTR and vasopressin V1 receptors in rat myometrial homogenates (Engstrom et al, 1998), its effects are likely mediated by OTR, since a recent study demonstrated antidepressant-like effects of the same dose via specific activation of the central OTR and not vasopressin V1A receptors (Chaviaras et al, 2010). Addressing emotional impairment associated with opioid abstinence is essential given the high comorbidity with depression along with the ambiguous efficacy of classic antidepressant treatment in this patient population (Nunes et al, 2004). In addition, the pro-social effect of CBT in morphine- withdrawn mice is consistent with previous reports demonstrating the key role of OT in the positive modulation of social behaviors (Lukas et al, 2011; Neumann et al, 2012). This is of particular interest as it represents the first attempt to modulate sociability deficits associated with opioid addiction. Although understudied, prolonged use of addictive substances often results in disintegration of the social lives due to social isolation and poor decision making at the expense of compulsive pre-occupation with the drug and its related cues (Dawe et al, 2009; Volkow et al, 2011). Considering the therapeutic success of social support programs, as well as the benefits of social rehabilitation and reintegration in keeping former addicts in a drug-free state (McGregor et al, 2012), the use of OT-ergic compounds in combination with psycho-social support may prove to be effective in preventing relapse by promoting positive social behavior. The pro-social effect of OT-ergic pharmacotherapy is being assessed in clinical trials for the treatment of social deficit in autistic spectrum disorders and schizophrenia (Carter, 2007; Heinrichs and Gaab, 2007). Interestingly, a decrease in the hypothalamic OT mRNA expression, as well as an increase in OTR binding in the amygdala, was observed in a mouse model of schizophrenia displaying social behavior deficits (Lee et al, 2005). Overall, these findings further support a causal relationship between the alterations observed within the OT peptidergic system and impaired social behavior.
In addition, CBT prevented stress-induced reinstatement of morphine CPP. Our findings are consistent with (Qi et al, 2009) who showed a beneficial effect of OT on attenuating reinstatement of methamphetamine CPP induced by restraint stress. This modulatory effect of CBT may involve regulation of drug reward as shown by the ability of OT to inhibit both methamphetamine- and cocaine-induced increase in dopamine utilization in the striatum (Kovacs et al, 1990; Qi et al, 2008). However, in our study, CBT, at 6.4 mg/kg (i.p.), was neither rewarding nor aversive in the CPP paradigm. An alternative mechanism underlying the effects of CBT may involve modulation of the HPA axis (Windle et al, 2004). However, in our study, carbetocin did not induce any differences in plasma corticosterone levels following stress-induced reinstatement. While these results suggest that the peripheral part of the HPA axis is not altered by CBT, its potential action via extra-hypothalamic brain stress systems (e.g., corticotropin-releasing factor, CRF) cannot be ruled out. In particular, the CRF system in the amygdala has been shown to play a key role in the aversive/emotional consequences of drug withdrawal (Erb, 2010; Heinrichs et al, 1995; Koob, 2009; Stinus et al, 2005) and stress-induced reinstatement of drug-seeking (Erb et al, 2001; Erb et al, 1998; Koob, 2009; Wang et al, 2006).
Taken together, our findings showed that the OT analogue CBT can prevent the negative emotional impairment, encompassing anxiety-, depressive-like behavior, reduction of sociability, as well as reinstatement of drug-seeking in a translational mouse model of opioid withdrawal. Future studies using opiate self-administration paradigms could be used to validate further these findings. Overall, our results highlight the alterations of the OT- ergic system as one of the underlying mechanisms of emotional impairment associated with long-term abstinence in opioid addicts. Thus, OT-ergic pharmacotherapy might constitute a novel potential target to assist opioid addicts with relapse prevention by treating mood disorders and social impairment associated with drug-withdrawal. The effects of chronic OT- ergic pharmacotherapy on the alleviation of negative emotional symptoms remain to be characterized to validate its progress towards clinical development.
Funding and Disclosures
This study was supported by Spyroula and Soteris Zanos, Lilia and Charalambos Georgiou and by a Royal Society grant (RG120556; P.I. Alexis Bailey). The authors declare that over the past three years P.Z. has received financial support from Mr and Mrs Zanos and P.G. has received financial support from Mr and Mrs Georgiou. The sponsors had no involvement in the design of the study and in the collection, analyses and interpretation of the data, nor in the writing of the report and the decision to submit this article for publication. Panos Zanos, Polymnia Georgiou, Sherie Wright, Susanna Hourani, Ian Kitchen, Raphaelle Winsky-Sommerer and Alexis Bailey report no conflict of interest and no biomedical financial interest from this research.
Acknowledgments
The authors thank Prof. Ingrid Nylander for valuable advice on OT peptide measurements, Dr. Sheryl Moy and Ms. Pamela Farshim for advice given on the behavioral tests, as well as Dr. van der Veen and Helen Keyworth for advice on statistical analyses.
Supplementary information is available at the Neuropsychopharmacology website
References
Anraku T, Ikegaya Y, Matsuki N, Nishiyama N (2001). Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology 157(2): 217-220.
Bailey A, Metaxas A, Al-Hasani R, Keyworth HL, Forster DM, Kitchen I (2010). Mouse strain differences in locomotor, sensitisation and rewarding effect of heroin; association with alterations in MOP-r activation and dopamine transporter binding. Eur J Neurosci 31(4): 742- 753.
Broadbear JH, Tunstall B, Beringer K (2011). Examining the role of oxytocin in the interoceptive effects of 3,4-methylenedioxymethamphetamine (MDMA, 'ecstasy') using a drug discrimination paradigm in the rat. Addict Biol 16(2): 202-214.
Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS (2010). Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology 58(1): 38-43.
Carter CS (2007). Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res 176(1): 170-186.
Chaviaras S, Mak P, Ralph D, Krishnan L, Broadbear JH (2010). Assessing the antidepressant-like effects of carbetocin, an oxytocin agonist, using a modification of the forced swimming test. Psychopharmacology 210(1): 35-43.
Christensson-Nylander I, Nyberg F, Ragnarsson U, Terenius L (1985). A general procedure for analysis of proenkephalin B derived opioid peptides. Regul Peptides 11(1): 65-76.
Cordery SF, Taverner A, Ridzwan IE, Guy RH, Delgado-Charro MB, Husbands SM, et al (2012). A non-rewarding, non-aversive buprenorphine/naltrexone combination attenuates drug-primed reinstatement to cocaine and morphine in rats in a conditioned place preference paradigm. Addict Biol. DOI: 10.1111/adb.12020.
Craft RM, Kostick ML, Rogers JA, White CL, Tsutsui KT (2010). Forced swim test behavior in postpartum rats. Pharmacol Biochem Behav 96(4): 402-412.
Dawe S, Davis P, Lapworth K, McKetin R (2009). Mechanisms underlying aggressive and hostile behavior in amphetamine users. Curr Opin Psychiatr 22(3): 269-273.
Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatr 62(10): 1187-1190.
Engstrom T, Barth T, Melin P, Vilhardt H (1998). Oxytocin receptor binding and uterotonic activity of carbetocin and its metabolites following enzymatic degradation. Eur J Pharmacol 355(2-3): 203-210.
Erb S (2010). Evaluation of the relationship between anxiety during withdrawal and stress- induced reinstatement of cocaine seeking. Prog Neuro-psychopharm 34(5): 798-807.
Erb S, Salmaso N, Rodaros D, Stewart J (2001). A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology 158(4): 360-365.
Erb S, Shaham Y, Stewart J (1998). The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci 18(14): 5529-5536.
Gimpl G, Fahrenholz F (2001). The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81(2): 629-683.
Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, et al (2011). Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry 69(3): 236-244.
Heinrichs M, Domes G (2008). Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res 170: 337-350.
Heinrichs M, Gaab J (2007). Neuroendocrine mechanisms of stress and social interaction: implications for mental disorders. Curr Opin Psychiatr 20(2): 158-162.
Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L (1995). Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol 6(1): 74-80.
Jaffe JH (1990). Trivializing dependence. Br J Addict 85(11): 1425-1427; Discussion 1429- 1431.
Keverne EB, Curley JP (2004). Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol 14(6): 777-783.
Koob G, Kreek MJ (2007). Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164(8): 1149-1159.
Koob GF (2008). A role for brain stress systems in addiction. Neuron 59(1): 11-34.
Koob GF (2009). Brain stress systems in the amygdala and addiction. Brain Res 1293: 61-75.
Kovacs CL, Van Ree JM (1985). Behaviorally active oxytocin fragments simultaneously attenuate heroin self-administration and tolerance in rats. Life Sci 37(20): 1895-1900.
Kovacs GL, Horvath Z, Sarnyai Z, Faludi M, Telegdy G (1985). Oxytocin and a C-terminal derivative (Z-prolyl-D-leucine) attenuate tolerance to and dependence on morphine and interact with dopaminergic neurotransmission in the mouse brain. Neuropharmacology 24(5): 413-419.
Kovacs GL, Izbeki F, Horvath Z, Telegdy G (1984). Effects of oxytocin and a derivative (Z- prolyl-D-leucine) on morphine tolerance/withdrawal are mediated by the limbic system. Behav Brain Res 14(1): 1-8.
Kovacs GL, Laczi F, Vecsernyes M, Hodi K, Telegdy G, Laszlo FA (1987). Limbic oxytocin and arginine 8-vasopressin in morphine tolerance and dependence. Exp Brain Res 65(2): 307- 311.
Kovacs GL, Sarnyai Z, Barbarczi E, Szabo G, Telegdy G (1990). The role of oxytocin- dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology 29(4): 365-368.
Le Moal M, Koob GF (2007). Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol 17(6-7): 377-393.
Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI (2005). Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology 30(10): 1883-1894.
Liu JCJ, McErlean RA, Dadds MR (2012). Are We There Yet? The Clinical Potential of Intranasal Oxytocin in Psychiatry. Curr Psychiatr Rev 8(1): 37-48.
Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID (2011). The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 36(11): 2159-2168.
Lukas M, Toth I, Veenema AH, Neumann ID (2013). Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology 38(6): 916-926.
Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H (2010). Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology 35(11): 2165-2178.
Martin WR, Jasinski DR (1969). Physiological parameters of morphine dependence in man-- tolerance, early abstinence, protracted abstinence. J Psychiatr Res 7(1): 9-17.
McGregor IS, Bowen MT (2012). Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav 61(3): 331-339.
McGregor IS, Callaghan PD, Hunt GE (2008). From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol 154(2): 358-368.
Muller DL, Unterwald EM (2004). In vivo regulation of extracellular signal-regulated protein kinase (ERK) and protein kinase B (Akt) phosphorylation by acute and chronic morphine. J Pharmacol Exp Ther 310(2): 774-782.
Neumann ID, Landgraf R (2012). Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 35(11): 649-659.
Nunes EV, Sullivan MA, Levin FR (2004). Treatment of depression in patients with opiate dependence. Biol Psychiatry 56(10): 793-802.
Peles E, Schreiber S, Naumovsky Y, Adelson M (2007). Depression in methadone maintenance treatment patients: rate and risk factors. J Affect Disord 99(1-3): 213-220.
Phelps EA, LeDoux JE (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48(2): 175-187.
Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF (2008). Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch Pharmacol 376(6): 441-448.
Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF (2009). Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology 56(5): 856-865.
Redila VA, Chavkin C (2008). Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology 200(1): 59-70.
Sarnyai Z (2011). Oxytocin as a potential mediator and modulator of drug addiction. Addict Biol 16(2): 199-201.
Sarnyai Z, Viski S, Krivan M, Szabo G, Kovacs GL, Telegdy G (1988). Endogenous oxytocin inhibits morphine tolerance through limbic forebrain oxytocin receptors. Brain Res 463(2): 284-288.
Singewald GM, Rjabokon A, Singewald N, Ebner K (2011). The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology 36(4): 793-804.
Stinus L, Cador M, Zorrilla EP, Koob GF (2005). Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology 30(1): 90-98.
Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, et al (2011). Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med 73(5): 393-400.
Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ (2010). A review of opioid dependence treatment: pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev 30(2): 155-166.
Volkow ND, Baler RD, Goldstein RZ (2011). Addiction: pulling at the neural threads of social behaviors. Neuron 69(4): 599-602.
Wang J, Fang Q, Liu Z, Lu L (2006). Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology 185(1): 19-28.
Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD (2004). Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci 24(12): 2974-2982.
You ZD, Li JH, Song CY, Wang CH, Lu CL (2000). Chronic morphine treatment inhibits oxytocin synthesis in rats. Neuroreport 11(14): 3113-3116.
Zanos P, Wright SR, Georgiou P, Yoo JH, Ledent C, Hourani S, et al (2013). Chronic methamphetamine treatment induces oxytocin receptor up-regulation in the amygdala and hypothalamus via an adenosine A2A receptor-independent mechanism. Pharmacol Biochem Behav. DOI: 10.1016/j.pbb.2013.05.009.
Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ (2006). Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol 191(1): 137-145.
Legends for Table and Figures
Table 1. Relevant effects for biochemical and behavioral data (two-way ANOVA)
Figure 1. Oxytocin receptor (OTR) binding, oxytocin (OT) peptide levels in the brain and plasma corticosterone levels following 7-day morphine abstinence. Male C57BL/6J mice were treated for seven days with either saline or chronic morphine followed by a withdrawal period of 7 days. (A) Representative autoradiograms of 50 pM [125I]-ornithine vasotocin analogue binding to OTRs in coronal brain sections at the level of the olfactory nuclei (row 1), striatum (row 2), septum (row 3) and thalamus (row 4). Binding levels are represented using a pseudo-colour interpretation of black and white film images in fmol/mg of tissue equivalent. (B) Quantitative OTR binding levels in brain regions where OTRs are expressed. (C) OT peptide levels in the septum and hypothalamus. (D) Corticosterone levels in plasma. Data are expressed as mean ± SEM (n=5-7 per group). *p<0.05; **p<0.01; ***p<0.001 (two-way ANOVA followed by Holm-Sidak post-hoc test when appropriate). Abbreviations: Acb, nucleus accumbens; Amy, amygdala; AOL, anterior olfactory nucleus- lateral; AOM, anterior olfactory nucleus-medial; AOV, anterior olfactory nucleus-ventral; CgCx, cingulate cortex; CPu, caudate putamen; Hip, hippocampus; Hyp, hypothalamus; LS, lateral septum; MS, medial septum; Pir, piriform cortex; PV Th, paraventricular nucleus of thalamus; Th, thalamus; Tu, olfactory tubercle; VDB, vertical limb of diagonal band of Broca.
Figure 2. Anxiety-like behavior induced by 7-day morphine withdrawal is reversed by the oxytocin analogue carbetocin (CBT). (A) Time spent in the open arms and (B) percentage of entries in the open arms of the elevated plus-maze. Seven-day withdrawal from chronic morphine treatment induced an increase in anxiety-like behavior which was reversed by CBT (6.4 mg/kg, i.p.). Data are expressed as mean ± SEM (n=6-10 per group). **p<0.01 (two-way ANOVA followed by Holm-Sidak post-hoc test when significance was reached). Abbreviations: Sal, saline; Mor, morphine.
Figure 3. Depressive-like behavior induced by 7-day morphine withdrawal is prevented by the oxytocin analogue carbetocin (CBT). (A) Immobility time, (B) latency to the first episode of immobility, and (C) number of fecal boli were measured during the 5-min forced- swim test. In 7-day morphine-withdrawn animals, immobility time and production of fecal boli were increased while the latency to the first episode of immobility was decreased in the forced-swim test, indicative of enhanced depressive-like behaviors. Administration of CBT (6.4 mg/kg, i.p.) reversed these effects of 7-day morphine withdrawal to levels comparable to the saline control group. Data are expressed as mean ± SEM (n=6-10 per group). *p<0.05; **p<0.01, ***p<0.001 (two-way ANOVA followed by Holm-Sidak post-hoc test when significance was reached). Abbreviations: Sal, saline; Mor, morphine.
Figure 4. Carbetocin (CBT) restores sociability behavior following 7-day morphine withdrawal using the three-chambered social approach test. (A) Sociability defined by the ratio of the time spent in the chamber containing the novel conspecific (“social chamber”) vs the empty chamber during the 10-min “sociability” phase. Mice were then tested for (B) preference for social novelty as defined by the ratio of the time spent in the chamber containing a novel unfamiliar mouse (“novelty chamber”) vs the chamber containing the former conspecific (“familiar chamber”) during the 10-min “preference for social novelty” phase. While mice withdrawn from chronic morphine treatment displayed a reduction in sociability compared to the saline-withdrawn group, CBT (6.4mg/kg, i.p.) restored sociability. Preference for social novelty was decreased in the chronic morphine-treated groups. Data are expressed as mean ± SEM (n=6-10 per group). *p<0.05, **p<0.01; ***p<0.001 (two-way ANOVA followed by Holm-Sidak post-hoc test when significance was reached). Abbreviations: Sal, saline; Mor, morphine.
Figure. 5. Effect of carbetocin (CBT) on stress-induced reinstatement of morphine- seeking behavior and conditioned place preference (CPP). (A) Experimental protocol with the different phases of the CPP paradigm. Twenty-six mice underwent the following protocol: Habituation phase; Pre-conditioning (Pre-Cond) phase: assessment of spontaneous place preference; Conditioning phase: 4 consecutive days with saline injection (10 ml/kg, s.c., first half of light period) in the preferred compartment and administration of morphine (10 mg/kg, s.c. 4 hours after the saline injection) in the least-preferred compartment; Post- conditioning (Post-Cond) session: assessment of conditioning with no injection; Extinction phase: 5 consecutive days with saline injection (10 ml/kg, s.c. first half of light period) and then, administration of saline (10 ml/kg, s.c. 4 hours after the first daily injection) in the drug- paired compartment; Post-extinction (Post-Ext) test: assessment of extinguished behavior - no injection; For the reinstatement test, mice were then subdivided into two experimental groups to receive either saline (4 ml/kg, i.p.; n=14) or CBT (6.4 mg/kg, i.p.; n=12), followed by exposure to a forced-swim stress 5 minutes later (23 ± 1oC; 6 min). Mice were then tested for reinstatement of morphine-seeking behavior during a 20-min session. (B) Time spent in the morphine-paired compartment for each phase of the CPP paradigm (analysis of the last 15 min of the 20-min session). Data are expressed as mean ± SEM (n=12-14 mice per group); *p<0.05; ***p<0.001. (C) Time spent in the CBT- or saline-paired compartment (analysis of the last 15 minutes of the 20-min session) during the pre-conditioning (Pre-Cond) and postconditioning (Post-Cond) phases of the CPP paradigm. Data are expressed as mean ± SEM (n=6 mice per group).