Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines
Joshua D. Dahlke, MD; Hector Mendez-Figueroa, MD; Lindsay Maggio, MD;
Alisse K. Hauspurg, MD; Jeffrey D. Sperling, MD; Suneet P. Chauhan, MD; Dwight J. Rouse, MD
OBJECTIVE: The purpose of this study was to compare 4 national guidelines for the prevention and management of postpartum hemorrhage (PPH).
STUDY DESIGN: We performed a descriptive analysis of guidelines from the American College of Obstetrician and Gynecologists practice bulletin, the Royal Australian and New Zealand College of Obstetricians and Gynaecologists, the Royal College of Obstetrician and Gynaecol- ogists (RCOG), and the Society of Obstetricians and Gynaecologists of Canada on PPH to determine differences, if any, with regard to defi- nitions, risk factors, prevention, treatment, and resuscitation.
RESULTS: PPH was defined differently in all 4 guidelines. Risk factors that were emphasized in the guidelines conferred a high risk of catastrophic bleeding (eg, previous cesarean delivery and placenta previa). All organizations, except the American College of Obstetrician and Gynecologists, recommended active management of the third stage of labor for primary prevention of PPH in all vaginal deliveries. Oxytocin was recommended universally as the medication of choice for PPH prevention in vaginal deliveries. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists and RCOG recommended development of a massive transfusion protocol to manage PPH resuscitation. Recommendations for nonsurgical treat- ment strategies such as uterine packing and balloon tamponade varied across all guidelines. All organizations recommended transfer to a tertiary care facility for suspicion of abnormal placentation. Specific indications for hysterectomy were not available in any guideline, with RCOG recommending hysterectomy “sooner rather than later” with the assistance of a second consultant.
CONCLUSION: Substantial variation exists in PPH prevention and management guidelines among 4 national organizations that high- lights the need for better evidence and more consistent synthesis of the available evidence with regard to a leading cause of maternal death.
Key words: guideline, management, postpartum hemorrhage, prevention
An important component of patient safety and the reduction of adverse outcomes includes the development of unambiguous guidelines.6 Previous comparisons of national guidelines on topics such as vaginal birth after cesarean delivery,7 intrapartum fetal surveillance,8 fetal growth restriction,9 and shoulder dystocia10 have high- lighted differences in definitions, cau- ses, and recommendations. Because PPH is a leading cause of maternal morbidity and death, synthesis of na- tional guidelines could inform schema to optimize peripartum outcomes. The purpose of this descriptive review is to compare 4 national guidelines and recommendations for 5 aspects of PPH: definition, risk factors, preven- tion, resuscitation, and treatment (nonsurgical and surgical).
MATERIALS AND METHODS
The American College of Obstetrician and Gynecologists (ACOG) practice bulletin on PPH, guidelines from the
Royal Australian and New Zealand Col- lege of Obstetricians and Gynaecologists (RANZOG), RCOG, and the Society of Obstetricians and Gynaecologists of Canada (SOGC) were accessed on July 1, 2014, and the data were compared. The following aspects of PPH were summa- rized: definition, risk factors, prevention, resuscitation, and treatment (nonsur- gical and surgical). Recommendations and strength of evidence were reviewed based on each guideline’s method of reporting. Finally, the references were compared with regard to the total number of randomized control trials, Cochrane reviews, and systematic re- views/metaanalyses that were cited. Institutional review board approval was exempted because of the descriptive na- ture of our study and analysis.
RESULTS
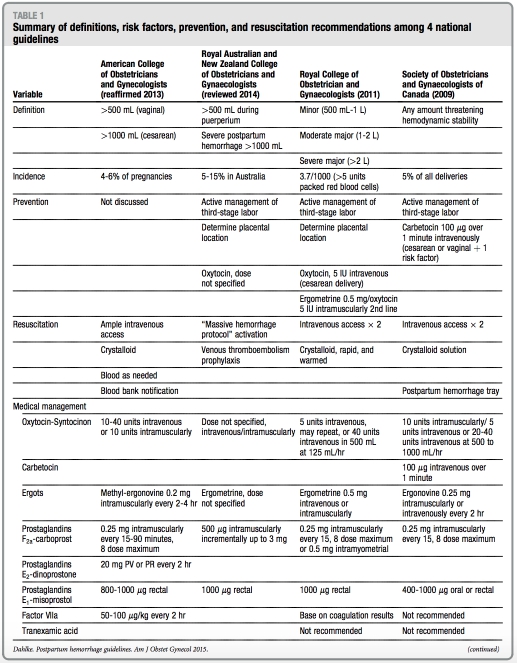
Definition
All of the guidelines used different defi- nitions of primary PPH. The ACOG practice bulletin defines PPH as blood loss of >500 mL for vaginal deliveries and >1000 mL for cesarean delivery. The RANZOG guideline defines PPH as >500 mL during puerperium and clas- sifies severe PPH as blood loss of >1000 mL. The RCOG guideline divides PPH into 3 categories: minor (500 mL to 1 L), moderate major (>1 L to 2 L), or severe major (>2 L). Finally, the SOGC guide- line is the only organization that defines PPH qualitatively: any amount of bleeding that threatens hemodynamic stability (Table 1).
Three guidelines (ACOG, RCOG, and SOGC) comment on the unreliability of estimated blood loss, such as using a visible estimate or through the use of blood collection drapes. None of the guidelines, however, recommended a preferred method to estimate blood loss. Despite the noted unreliability, estimates of blood loss nonetheless are used to initiate levels of treatment in RCOG guidelines. For example, minor PPH (500 mL to 1 L) should prompt basic measures such as intravenous access, indwelling bladder catheterization, full blood count and type, and screen; major PPH (estimated blood loss, >1 L) prompts a treatment protocol to achieve full resuscitation.
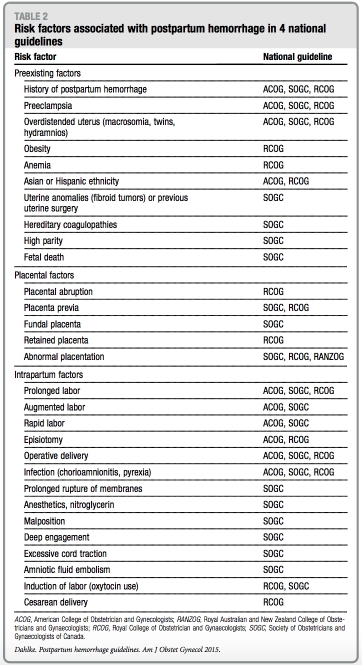
Risk factors
Risk factors described in the guidelines are summarized in Table 2. All guidelines note that most women who experience estimate of what proportion of women PPH do not have any known risk factors; with PPH are without risk factors. The none of the guidelines provide an RCOG guideline is the only 1 that provides approximate odds ratios (OR) for various risk factors. Those identified as highest risk include women with suspected or proven placental abruption (OR, 13; 99% CI, 7.6e12.9), known placenta previa (OR, 12; 99% CI, 7.2e23), multiple pregnancy (OR, 5; 99% CI, 3.0e6.6), and preeclampsia/ gestational hypertension (OR, 4; 99% CI, not specified), with delivery in a consultant-led maternity unit advised for women with these risk factors.
Women at risk for abnormal placen- tation and subsequent hemorrhage (such as those with a history of cesarean delivery and placenta previa) are dis- cussed specifically in all 4 guidelines. RANZOG and SOGC guidelines re- commend antenatal assessment of placentation and location in these high- risk women to prompt transfer to a tertiary care center or unit with rapid access to blood products or an intensive care unit. In addition, ACOG and RCOG guidelines recommend patient counseling about the likelihood of hys- terectomy and blood transfusion, the availability of blood products, and cell-saver technology and encourage planned delivery with preoperative anesthesia assessment. None of the guidelines specify the preferred modality for evaluation of abnormal placentation (eg, ultrasound vs magnetic resonance imaging).
Prevention
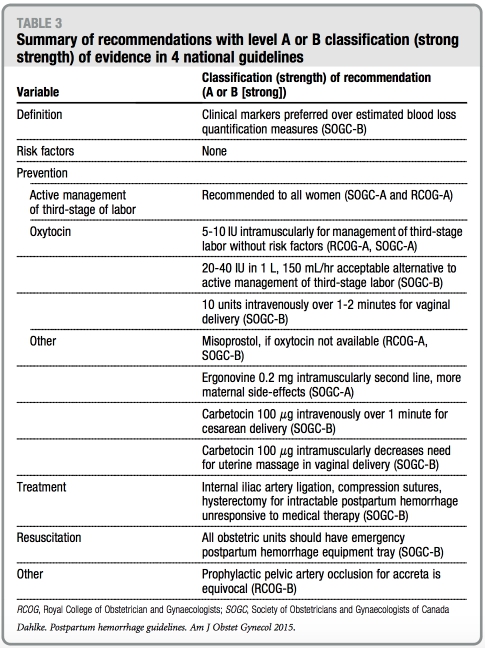
There are no specific recommendations discussed in any of the guidelines with regard to PPH prevention strategies before the onset of the third stage of la- bor. All guidelines, with the exception of ACOG, discuss active management of the third stage of labor (AMTSL) with strong recommendations for its use in primary prevention of PPH. AMTSL traditionally involves 3 interventions that are designed to assist in placenta expulsion: uterotonics, immediate um- bilical cord clamping, and controlled cord traction. Despite strong recom- mendation of this practice, RCOG and SOGC guidelines separate and stratify these interventions and recommend delayed cord clamping for neonatal benefitwhenfeasible.
Oxytocin is recommended universally as the first-line uterotonic of choice for prevention of uterine atony. ACOG and RANZOG guidelines do not specify dosing or route of administration. The RCOG guideline recommends 10 units intramuscularly for uncomplicated vaginal deliveries and 5 IU intravenous slow infusion after cesarean delivery. Finally, the SOGC guideline recom- mends different uterotonic medications depending on the clinical scenario. For example, oxytocin 10 units intramuscu- larly or 5-10 units intravenously over 1-2 minutes is recommended for low-risk vaginal deliveries; carbetocin 100 mg intravenously over 1 minute is recom- mended for cesarean delivery or vaginal delivery in women with 1 risk factor for PPH. Carbetocin, a oxytocin analogue with a significantly longer half-life than endogenous or synthetic oxytocin, is available in the United Kingdom, Ireland, Canada, Australia, and New Zealand, but not the United States.11 Misoprostol is recommended by the RANZOG guideline as a second-line preventive medication or when oxy- tocin is not available for PPH preven- tion; SOGC guidelines recommends ergonovine as a second-line agent or when oxytocin is not available. Synto- metrine at a fixed dose combination of 5 IU oxytocin and 0.5 mg ergometrine is recommended by the RCOG guideline as second-line prophylactic agents if avail- able and emphasizes the higher side- effect profile of this medication.
Resuscitation
All 4 guidelines discuss resuscitative measures during PPH with emphasis on fluid management and indications for blood products. A multidisciplinary approach with strong communication with anesthesia is recommended strongly. Although the SOGC guideline suggests that institutions develop and make available specific PPH trays, RANZOG advocates institutional devel- opment of a massive transfusion proto- col in cases of severe PPH, and that guideline is the only one that provides a massive transfusion protocol algori- thm template. Cell-saver technology or autologous transfusion is discussed briefly in ACOG and RCOG guidelines to assist in resuscitative efforts.
Treatment
Treatment modalities, when PPH is identified, can be categorized as nonsurgical or surgical. In general, there is large variation among guidelines with regard to PPH treatment. Notably, all guidelines, except RANZOG, recom- mend instituting a policy or establishing a protocol when PPH is identified, yet the specifics to the protocol vary or are not established. Regarding unique nonsurgical management options, the RCOG guideline discusses pneumatic antishock gear as a temporizing measure if available, although does not specify when, in the management schema, it should be used.
Tranexamic acid, an antifibrinolytic amino acid derivative of lysine, is discussed only in RCOG guidelines. Although shown to decrease bleeding significantly in nonobstetric procedures, particularly in trauma, RCOG recom- mends against its use. Similarly, another antifibrinolytic medication, recombi- nant factor VIIa, is mentioned in ACOG, RCOG, and SOGC guidelines. It is dis- cussed extensively in the ACOG guide- line; however, indications for its use are not specified. In contrast, recombinant factor VIIa is not recommended in SOGC and RCOG guidelines as a medi- cal treatment option for PPH.
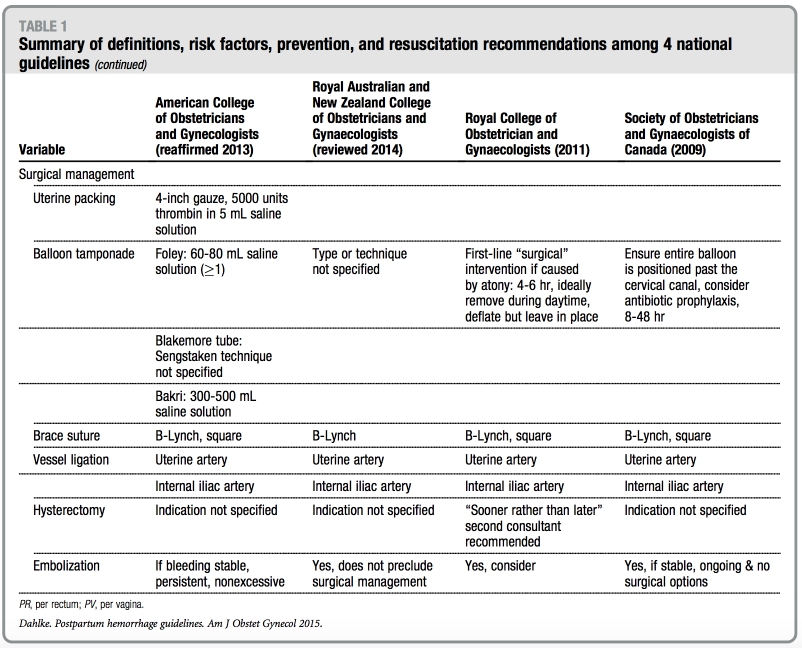
All guidelines discuss 8 surgical tech- niques: (1) uterine packing, (2) balloon tamponade, (3) uterine curettage, (4) uterine artery ligation, (5) brace suture, (6) hypogastric artery ligation, (7) arterial embolization, and (8) hysterec- tomy. In general, less invasive fertility- sparing interventions are promoted. The SOGC guideline is the only 1 that provides figures of both B-Lynch and Cho compression suture techniques. The ACOG guideline is the only
guideline that discusses the management of hemorrhage because of a ruptured uterus or inverted uterus. With regard to hysterectomy, the RCOG guideline em- phasizes early recourse to hysterectomy and not delaying this decision until the woman is in extremis and further rec- ommends subtotal hysterectomy, unless trauma to the lower uterine segment or cervix is noted. Additionally, the SOGC guideline notes that indications for hys- terectomy include massive hemorrhage that is not responsive to previous in- terventions and that the surgical inter- vention chosen should be familiar to surgeons.
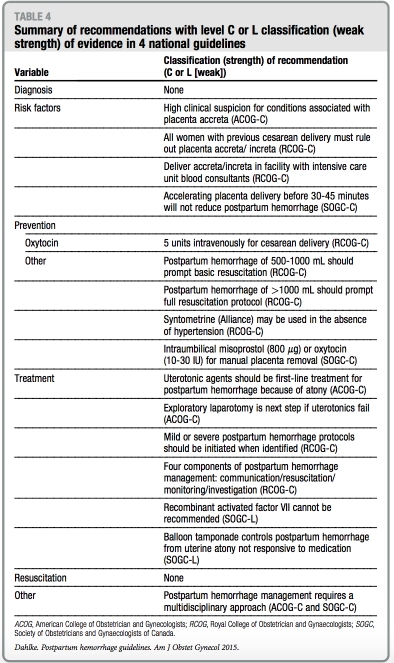
Tables 3 and 4 summarize all recom- mendations by each respective national guideline with regard to the classification or strength of evidence. Notably, none of the recommendations with either strong or weak strength of evidence are endorsed by >2 of the national guide- lines that were reviewed.
References
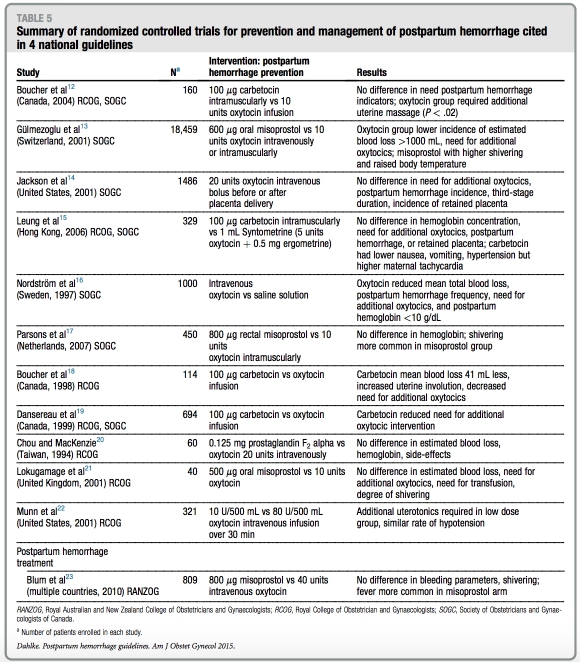
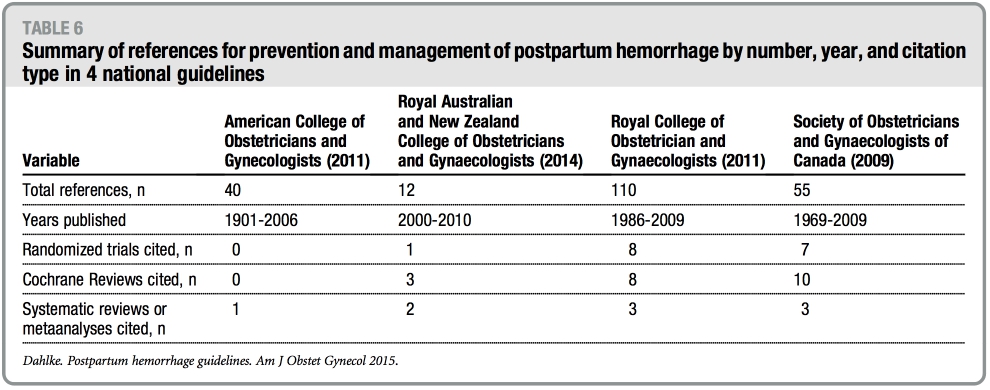
The number of references cited in each guideline ranges from 12 (RANZOG) to 110 (RCOG) with publication years be- tween 1901 through 2010. Table 5 sum- marizes the randomized controlled trials referenced with regard to PPH preven- tion or treatment in the setting of vaginal or cesarean delivery.12-23 Finally, Table 6 summarizes the number of randomized controlled trials, Cochrane reviews, and systematic reviews referenced in the guidelines. Notably, the ACOG practice bulletin does not cite a single random- ized controlled trial or Cochrane review in its guideline.
COMMENT
Recent epidemiologic studies note that PPH, particularly because of uterine atony, is increasing in the United States and abroad and that it is the major cause of obstetric morbidity and death in the world.24-26 Recommendations in the 4 national guidelines reviewed herein suggest significant differences in how this common complication is defined, anticipated, prevented, and treated. Also notable is the types of studies that have been used to make recommendations, which suggests variation in the methods
of the respective organizations’ devel- opment of practice guidelines.
The variation among the guidelines reviewed with regard to how PPH is defined is worth highlighting. Part of this difficulty may be due to the difficulty and inaccuracies of estimating blood loss. Efforts such as the quantification of blood loss proposed by the Association of Women’s Health, Obstetric and Neonatal Nurses potentially may improve the accuracy of blood loss estimation and subsequently improve the definition of PPH.27 Although our review compared the definition of PPH from 4 guidelines, we should also note that other definitions of PPH have been developed. For example, the ACOG
reVITALize initiative has developed the following definition of early PPH in the United States: cumulative blood loss of at least 1000 mL or blood loss accompanied by sign/symptoms of hypovolemia within 24 hours after the birth process (includes intrapartum loss).28 This contrasts with the World Health Organization definition of blood loss of !500 mL within 24 hours after birth.2 Finally, a recent international expert panel defined persistent (ongoing) PPH as “active bleeding >1000 mL within 24 hours after birth that continues despite the use of initial measures that include first-line uterotonic agents and uterine massage,” which highlights the clinical importance of the identification of bleeding that continues despite preventa- tive strategies.29
Women at high risk for abnormal placentation understandably are empha- sized in all of the national guidelines. Although most PPH cannot be predicted, this is one clinical scenario in which, at least for some women, the risk is known and can be anticipated. Appropriate planning of delivery from timing to location, with transfer to a tertiary hos- pital as needed, is paramount. Notably, specific PPH prevention strategies are not mentioned in the ACOG guideline, despite significant emphasis in the RANZOG, RCOG, and SOGC guidelines. All of the guidelines, however, recom- mend institutional drills and/or protocols to prepare for this inevitable event.
Initiatives such as the National Part- nership for Maternal Safety and the California Maternal Quality Care Collaborative identified obstetric hem- orrhage appropriately as a key priority in improving maternal safety and pro- vided recommendations, resources, and education to assist in this goal.30,31 For example, the obstetric hemorrhage core bundle proposed by D’Alton et al30 recommends the following for all US birthing facilities: (1) a standardized obstetric hemorrhage protocol and event checklist, (2) a hemorrhage kit or cart with appropriate medication and equipment, (3) a partnership with the local blood bank for rapid and sustained availability of blood products, and (4) the universal use of AMTSL.30 Similarly, the California Maternal Quality Care Collaborative offers an obstetric hem- orrhage toolkit that consists of (1) a compendium of best practices, (2) care guidelines with checklists, flowcharts, and table charts, (3) a hospital-level implementation guide, and (4) a slide set for professional education.31 Although it might seem self-evident that these ini- tiatives will improve PPH outcomes, nevertheless they should be evaluated prospectively.
Despite the emphasis on patient safety and institutional quality improvement, a recent survey of academic US obstetric units demonstrated at least 20% did not have a PPH protocol.32 Similarly, out- comes that have been associated with the implementation of massive transfusion protocols for severe PPH have been shown to be favorable, yet such protocols are explicitly recommended only by RANZOG.33 Future studies undoubtedly will shed light on optimal resuscitation and should be reflected in updated national guidelines.
AMTSL remains a recommended and highly studied preventive strategy for PPH. Recent studies, however, suggest that the major driver of this preventive strategy’s effectiveness is the adminis- tration of oxytocin. In a recent multi- center randomized controlled trial in 5 maternity units in France, Deneux- Tharaux et al34 found that controlled cord traction made minimal contribu- tion to overall blood loss in high resource settings in those who received oxytocin. In addition, an increasingly large body of evidence suggests that delayed cord clamping may have beneficial neonatal outcomes (improved long-term iron stores and hemoglobin concentration) without increasing the risk of maternal hemorrhage.35 These data suggest that, of the 3 interventions classically de- scribed in AMTSL (oxytocin, immediate cord clamping, controlled cord trac- tion), oxytocin, and oxytocin alone, re- mains the most important intervention for the prevention of PPH.
Research is ongoing to determine the optimal dose, route, and timing of the administration of oxytocin, but it remains the first-line medication for PPH prevention.36 Randomized trials of newer medications such as carbetocin or ranexamic acid have been conduct- ed,37,38 but additional studies are necessary to determine what role, if any, these medications should play in PPH prevention or management.
As evidenced by the paucity of ran- domized controlled trials that compare different medical treatment strategies for PPH or optimal surgical interventions, the order for which these management options are to be used remain vastly understudied. Although interventions such has balloon tamponade have made their way into management protocols and guidelines, there has yet to be a randomized clinical trial performed to compare it with any other treatment strategy.39
The strengths of our analysis include synthesizing the major guideline rec- ommendations on all aspects of PPH including definitions, risk factors, resuscitation, and medical and surgical management. In addition, we believe a critical evaluation of how the available evidence from randomized controlled trials, systematic reviews, and meta- analyses is used to develop these guidelines may improve future guide- lines and recommendations. We also recognize weaknesses in our descriptive analysis. First, we limited our review to 4 English language national guide- lines, despite other countries and orga- nizations such as the World Health Organization producing similar guide- lines for the prevention and manage- ment of PPH.2 Our goal in doing this was to compare and contrast recom- mendations whose guidelines have been previously compared7-10 and are germane to similarly resourced settings with similar intended audiences con- cerning this important topic. We also acknowledge that the authors or com- mittees who developed the guidelines that we reviewed may be subject to differing methods for establishing rec- ommendations within each national organization.
In summary, PPH universally remains a major cause of maternal morbidity and death in both developed and devel- oping countries. As the evidence-base for PPH and management improves, a convergence of national guidelines ought to occur to reflect best available practices.
REFERENCES
1. American College of Obstetricians and Gy- necologists. Clinical management guidelines for obstetrician-gynecologists: postpartum hemor- rhage. ACOG Practice bulletin no. 76. Obstet Gynecol 2006;108:1039-47.
2. World Health Organization. WHO recommen- dations for the prevention and treatment of postpartum haemorrhage. Geneva: World Health Organization. 2012. WHO Guidelines Approved by the Guidelines Review Committee. Available at: http://www.who.int/reproductivehealth/publi cations/maternal_perinatal_health/97892415 48502/en/. Accessed Nov. 1, 2013.
3. Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Management of postpartum hemorrhage. March 2011. Available at: http://www.ranzcog.edu.au/college- statements-guidelines.html. Accessed Nov. 1, 2013.
4. Leduc D, Senikas V, Lalonde AB, et al. Active management of the third stage of labour: pre- vention and treatment of postpartum hemor- rhage. J Obstet Gynaecol Can 2009;31:980-93. 5. Royal College of Obstetrician and Gynaecol- ogists. Postpartum hemorrhage: prevention and management. April 2011. Available at: http:// www.rcog.org.uk/womens-health/clinical-gui dance/prevention-and-management-postpart um-haemorrhage-green-top-52. Accessed Nov. 1, 2013.
6. Clark SL, Belfort MA, Byrum SL, Meyers JA, Perlin JB. Improved outcomes, fewer cesarean deliveries, and reduced litigation: results of a new paradigm in patient safety. Am J Obstet Gynecol 2008;199:105.e1-7.
7. Hill JB, Ammons A, Chauhan SP. Vaginal birth after cesarean delivery: comparison of ACOG practice bulletin with other national guidelines. Clin Obstet Gynecol 2012;55:969-77.
8. Hill JB, Chauhan SP, Magann EF, Morrison JC, Abuhamad AZ. Intrapartum fetal surveillance: review of three national guidelines. Am J Perinatol 2012;29:539-50.
9. Chauhan SP, Gupta LM, Hendrix NW, Berghella V; American College of Obstetricians and Gynecologists. Intrauterine growth restric- tion: comparison of American College of Ob- stetricians and Gynecologists practice bulletin with other national guidelines. Am J Obstet Gynecol 2009;200:409.e1-6.
10. Chauhan SP, Gherman R, Hendrix NW, Bingham JM, Hayes E. Shoulder dystocia: comparison of the ACOG practice bulletin with another national guideline. Am J Perinatol 2010;27:129-36.
11. Ferring Pharmaceuticals. Products. Available at: http://www.ferringusa.com/products. Accessed Dec. 14, 2013.
12. Boucher M, Nimrod CA, Tawagi GF, et al. Comparison of carbetocin and oxytocin for the prevention of postpartum hemorrhage following vaginal delivery: a double-blind randomized trial. J Obstet Gynaecol Can 2004;26:481-8.
13. Gülmezoglu AM, Villar J, Ngoc NT, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet 2001;358:689-95.
14. Jackson KW Jr, Allbert JR, Schemmer GK, Elliot M, Humphrey A, Taylor J. A randomized controlled trial comparing oxytocin administration before and after placental delivery in the preven- tion of postpartum hemorrhage. Am J Obstet Gynecol 2001;185:873-7.
15. Leung SW, Ng PS, Wong WY, Cheung TH. A randomised trial of carbetocin versus synto- metrine in the management of the third stage of labour. BJOG 2006;113:1459-64.
16. Nordström L, Fogelstam K, Fridman G, Larsson A, Rydhstroem H. Routine oxytocin in the third stage of labour: a placebo controlled randomised trial. BJOG 1997;104:781-6.
17. Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Rectal misoprostol versus oxytocin in the management of the third stage of labour. J Obstet Gynaecol Can 2007;29:711-8.
18. Boucher M, Horbay GL, Griffin P, et al. Double-blind, randomized comparison of the effect of carbetocin and oxytocin on intra- operative blood loss and uterine tone of patients undergoing cesarean section. J Perinatol 1998;18:202-7.
19. Dansereau J, Joshi AK, Helewa ME, et al. Double-blind comparison of carbetocin versus oxytocin in prevention of uterine atony after ce- sarean section. Am J Obstet Gynecol 1999;180: 670-6.
20. Chou MM, MacKenzie IZ. A prospective, double-blind, randomized comparison of pro- phylactic intramyometrial 15-methyl prosta- glandin F2 alpha, 125 micrograms,and intravenous oxytocin, 20 units, for the control of blood loss at elective cesarean section. Am J Obstet Gynecol 1994;171:1356-60.
21. Lokugamage AU, Paine M, Bassaw- Balroop K, Sullivan KR, Refaey HE, Rodeck CH. Active management of the third stage at caesarean section: a randomised controlled trial of misoprostol versus syntocinon. Aust N Z J Obstet Gynaecol 2001;41:411-4.
22. Munn MB, Owen J, Vincent R, Wakefield M, Chestnut DH, Hauth JC. Comparison of two oxytocin regimens to prevent uterine atony at cesarean delivery: a randomized controlled trial. Obstet Gynecol 2001;98:386-90.
23. Blum J, Winikoff B, Raghavan S, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet2010;375:217-23.
24. Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol 2013;209:449.e1-7.
25. Mehrabadi A, Liu S, Bartholomew S, et al. Temporal trends in postpartum hemorrhage and severe postpartum hemorrhage in Canada from 2003 to 2010. J Obstet Gynaecol Can 2014;36: 21-33.
26. Lutomski JE, Byrne BM, Devane D, Greene RA. Increasing trends in atonic postpartum haemorrhage in Ireland: an 11-year population-based cohort study. BJOG 2012;119: 306-14.
27. Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN). Practice brief number 1: quantification of blood loss. Available at: http://www.pphproject.org/downloads/awho nn_qbl.pdf. Accessed: December 27, 2014.
28. American Congress of Obstetricians and Gynecologists. reVITALize obstetric data defini- tions. Available at: http://www.acog.org/-/media/ Departments/Patient-Safety-and-Quality-Improve ment/2014reVITALizeObstetricDataDefinitionsV10. pdf. Accessed Dec. 27, 2014.
29. Abdul-Kadir R, McLintock C, Ducloy AS, et al. Evaluation and management of postpartum hemorrhage: consensus from an international expert panel. Transfusion 2014;54:1756-68. 30. DʼAlton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol 2014;123:973-7.
31. California Maternal Quality Care Collabora- tive. OB hemorrhage toolkit. Available at: https:// cmqcc.org/ob_hemorrhage. Accessed Dec. 27, 2014.
32. Kacmar RM, Mhyre JM, Scavone BM, Fuller AJ, Toledo P. The use of postpartum hemorrhage protocols in United States aca- demic obstetric anesthesia units. Anesth Analg 2014;119:906-10.
33. Gutierrez MC, Goodnough LT, Druzin M, Butwick AJ. Postpartum hemor- rhage treated with a massive transfusion protocol at a tertiary obstetric center: a retrospective study. Int J Obstet Anesth 2012;21:230-5.
34. Deneux-Tharaux C, Sentilhes L, Maillard F, et al. Effect of routine controlled cord traction as part of the active management of the third stage of labour on postpartum haemorrhage: multi- centre randomised controlled trial (TRACOR). BMJ 2013;346:f1541.
35. McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev 2013;7:CD004074.
36. Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of la- bour to prevent postpartum haemorrhage. Cochrane Database Syst Rev 2013;10: CD001808.
37. Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2012;4:CD005457.
38. Novikova N, Hofmeyr GJ. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2010;7: CD007872.
39. Wright CE, Chauhan SP, Abuhamad AZ. Bakri balloon in the management of postpartum hemorrhage: a review. Am J Perinatol 2014;31: 957-64.
