Differing In Vitro Potencies of Tocolytics and Progesterone in Myometrium From Singleton and Twin Pregnancies
Sarah Arrowsmith, PhD1, James Neilson, MD2, Leanne Bricker, MRCOG3,4, and Susan Wray, PhD1
Abstract
We compared the relaxant effect of 2 known tocolytics; indomethacin and atosiban and progesterone, on pregnant human myometrial spontaneous and oxytocin-induced contractions from singleton and twin pregnancies. All agents exerted a concentration-dependent relaxant effect on myometrial contractions. There was no significant difference in the concentration– response curves between singletons and twins for progesterone or indomethacin on spontaneous contractions or atosiban on oxytocin-induced contraction. Under oxytocin however, the concentration–response curves for indomethacin and progesterone were significantly shifted to the right for both amplitude of contraction (P < .01) and activity integral (P < .01). When compared to singleton myometrium however, the concentration–response curves were significantly shifted to the right in the twin myome- trium group (P < .05 progesterone and P < .001 indomethacin). We conclude that a greater concentration of progesterone and indomethacin is required to inhibit oxytocin-induced myometrial contractions in twins compared to singletons in vitro. The differences noted in the tissue pharmacologies may have implications for the successful prevention or inhibition of preterm labor in twin pregnancy.
Keywords
human myometrium, oxytocin, progesterone, tocolytics, twin pregnancy
Introduction
Preterm birth, defined as delivery before 37 weeks of gestation affects between 5% and 18% of pregnancies worldwide and accounts for 70% of neonatal morbidity and mortality.1 Whilst twin pregnancies represent just 1% to 3% of births, multiple gestation, that is, twins and other higher order pregnancies con- tribute disproportionately to preterm birth rates with at least 10% of preterm births being attributed to twins2 and at least 25% of admissions to neonatal units are from multiple pregnan- cies. As a result, multiple pregnancies are considered as at high risk of preterm delivery.
The causes of spontaneous preterm birth are diverse and include a number of pathological processes such as infection, antepartum hemorrhage, and cervical insufficiency but its etiol- ogy is likely to be multifactorial having contributions from the fetus, fetal membranes, and the myometrium.3,4 In the case of twin pregnancy, the mechanism for preterm birth is not clear, however, uterine overdistension resulting from increased fetal number (and membranes) has been widely implicated since myometrial stretch increases myometrial contractility, upregu- lates oxytocin receptors, and prostaglandin release, all of which contribute to the cascade of events leading to labor onset.5 We have found differences in myometrial contractility in vitro at
term in twins compared to singletons, with increased frequency and increased response to oxytocin,6 further supporting the suggestion that mechanisms disposing to preterm labor are present in twin pregnancy myometrium.
As myometrial contractions are one of the most recogniz- able signs of preterm labor, clinical research has focused toward the development and use of tocolytics to pharmacologi- cally inhibit or reduce uterine contractions to prevent preterm delivery. However, no particular tocolytic agent has yet proven optimal.7 Clinical experience of tocolytic use in the setting of twin pregnancies is also limited; they are rarely used except for in utero transfer. The most studied tocolytic drugs in twin gestations are betamimetics but there is insufficient evidence to support their use in women with twins.8
In singleton pregnancy, a number of trials have shown antena- tal progestogen therapy (vaginally administered natural proges- terone or the synthetic progestogen 17-hydroxyprogesterone caproate), given prophylactically, in women at high risk of pre- term delivery because of history of prior preterm delivery or with sonographically short cervix, to be effective in reducing preterm birth,9-13 but this has not been found in twins10,14-18 or triplets.19,20 Compared to singleton pregnancy, the role of other agents such as indomethacin and oxytocin receptor antagonists has not been extensively studied or systematically compared in twin gestations and currently, no treatment has been identified to prevent preterm birth in twins.3
The aim of this present study was to investigate the poten- cies of 2 widely used tocolytics: (1) the oxytocin receptor antagonist atosiban and (2) the cyclooxygenase (COX) inhibi- tor indomethacin as well as the acute actions of the steroid hor- mone progesterone on in vitro myometrial contractions from women with singleton or twin pregnancy. Atosiban and indo- methacin were chosen for their use clinically as acute tocolytics, while progesterone was chosen for its suggested prophylactic properties and following the differences noted in its ability to prevent preterm delivery in a select group of singleton but not twin gestations.
Materials and Methods
Tissue Collection and Preparation
Sixty-four biopsies of human myometrium were obtained from women with singleton (n 1⁄4 26) or twin pregnancies (n 1⁄4 38) undergoing prelabor elective cesarean section (CS) between 31 weeks þ3 days and 41 weeks gestation at Liverpool Women’s Hospital (LWH), Liverpool, United Kingdom. All sections were performed before the onset of labor for the following reasons: previous CS (n 1⁄4 15), breech (n 1⁄4 11), maternal request (n 1⁄4 11), previous difficult vaginal delivery (n 1⁄4 9), fetal reason, for example, intrauterine growth restriction (IUGR) (n 1⁄4 9), other maternal reason (n 1⁄4 7), and placenta previa (n 1⁄4 2). Approval by the North West-Liverpool East Research Ethics Committee (institutional review board) was granted (REC ref 10/H1002/ 49) and all women gave written informed consent to participate. Full details of the demographics for all women are given in Table 1A and term-only women in Table 1B. As expected there were differences in fetal weight (combined weight in case of twins) and gestational age. Women with twin pregnancy also tended to have a lower parity (higher percentage of nul- liparity) and required CS for fetal reasons, breech presenta- tion, or maternal request, while women with singleton women tended to be higher parity and requiring CS for previous CS or previous traumatic delivery. At surgery, biopsies (around 1 2 cm2) were cut from the upper lip of the lower uterine inci- sion site and placed into Hanks’ balanced salt solution.21
1 Department of Cellular and Molecular Physiology, Harris-Wellbeing Preterm Birth Centre, Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom
2 Department of Women and Children’s Health, Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom
3 Liverpool Women’s NHS Foundation Trust, Liverpool, United Kingdom
4 Current address: Corniche Maternity Hospital, Abu Dhabi, United Arab Emirates
Table 1A. Maternal Demographics of Women Delivering by CS According to Pregnancy Group (All Gestations).a
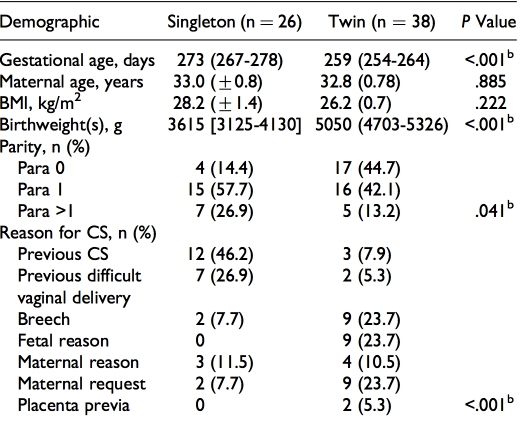
Abbreviations: BMI, body mass index; CS, cesarean section; IQR, interquartile range; SEM, standard error of the mean.
aData are presented as mean (+SEM), median (IQR), or frequencies (counts, n, and percentages) where appropriate. Gestational age and maternal age are age at delivery. The BMI was recorded at pregnancy booking. In the case of twins, the combined birthweights of both babies are given. bSignificant difference (P < .05) by unpaired t test, Mann-Whitney U test, or chi- square (w2) test.
Table 1B. Maternal Demographics of Women Delivering by CS at Term According to Pregnancy Group.a
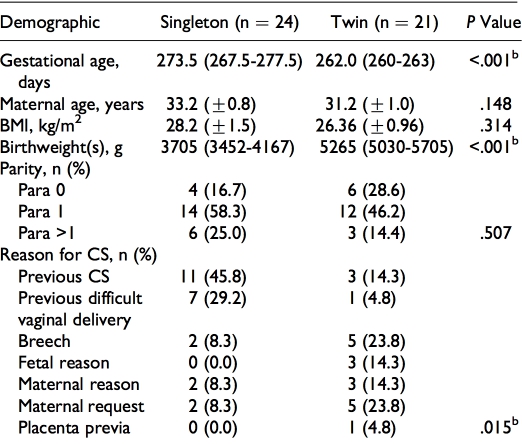
Abbreviations: BMI, body mass index; CS, cesarean section; IQR, interquartile range; SEM, standard error of the mean.
aData are presented as mean (+SEM), median (IQR), or frequencies (counts, n, and percentages) where appropriate. Gestational age and maternal age are age at delivery. The BMI was recorded at pregnancy booking. In the case of twins, the combined birthweights of both babies are given. bSignificant difference (P < .05) by unpaired t test, Mann-Whitney U test, or chi- square (w2) test.
Samples for experimentation were used immediately upon col- lection or within 12 hours of collection with storage at 4 C.
In the laboratory, multiple strips of myometrium from each participant cleared of uterine decidua, serosa, and scar tissue and measuring approximately 5 mm long, 2 mm wide, and 1 mm thick, following the plane of the muscle fibers, were cut from adjacent sites within each biopsy. Strips were mounted horizontally using aluminum clips within 1 mL organ baths for isometric recording under 2 mN of resting tension as previously described.6,22 Strips were continually superfused with physio- logical saline solution (PSS, in mmol/L: 154 NaCl, 5.6 KCl, 1.2 MgSO4, 7.8 glucose, 10.9 HEPES, and 2.0 CaCl2) at a rate of 1.5 mL/min, pH 7.4 at 36 C and were left to equilibrate under these conditions for at least 2 hours or until a steady base- line of contractile activity was observed, typically within 3 hours. Where no spontaneous activity was achieved follow- ing 3 hours of superfusion with PSS, the strip was discarded.
Concentration–Response Experiments
Preliminary studies were performed to plan concentration ranges for testing and to determine vehicle suitability by way of time-matched vehicle control experiments and optimal experimental length to control for potential tissue fatigue. For spontaneous contractions, myometrial strips were left to equili- brate for 1 hour after the onset of contractions or until a mini- mum of 4 consecutive contractions of equal amplitude and regular frequency were achieved. For oxytocin-induced con- tractions, myometrial strips were allowed to equilibrate as previously, after which contractions were stimulated with oxy- tocin (0.5nmol/L) for a minimum of 45 minutes to achieve a stable baseline activity under stimulation. Strips were then exposed to rising concentrations of each drug, added at 20 min- ute intervals. Progesterone was examined at concentrations of 1, 10, 50, and 100 mmol/L, indomethacin was examined at con- centrations of 10, 20, 30, 40, and 50 mmol/L, and atosiban was examined at concentrations of 1 nmol/L, 10 nmol/L, 100 nmol/L, 1 mmol/L, and in some cases 10 mmol/L was also examined. In the case of oxytocin-induced contractions, all drugs were applied in the continued presence of 0.5 nmol/L oxytocin.
In total, 49 different biopsies were used for the analysis of the effect of progesterone or indomethacin on spontaneous con- tractility or atosiban on oxytocin-induced contractions. Where possible, 1 strip from each biopsy was used to test each agent (or control) thus; drugs were tested in parallel from each patient. No patient provided more than 1 strip for each agent. Experiments were performed until a minimum of 20 data sets representing 20 different women for each tocolytic were col- lected. A further 15 biopsies were used in the analysis of the effects of progesterone or indomethacin under stimulation with oxytocin until a data set representing at least 8 different women for each tocolytic or progesterone and pregnancy group (single- ton or twin) was achieved. For all experiments, the concentra- tion of vehicle (ethanol or dimethyl sulfoxide [DMSO] for progesterone and indomethacin experiments, respectively) did not exceed 0.1% (v/v).
Data Collection and Analysis
Data were collected at a sampling rate of 10 Hz via FORT-10g transducers connected to a 4-channel transbridge amplifier and data acquisition hardware unit running the associated software (Labscribe) all purchased from World Precision Instruments, Hertfordshire, United Kingdom and was analyzed using Origin Pro9.0 Software [OriginLab, USA].23 Measurement of contrac- tile activity was performed by calculation of the integral area under the tension curve (AUC, arbitrary units) and mean max- imum amplitude of contraction (expressed in mN). Contractile activity in the final 20 minutes preceding the addition of the first concentration was used as control activity. The effects of each drug at each concentration were similarly calculated and expressed as a percentage of the integral and amplitude during the control period (ie, control activity is equal to 100%).
Concentration–response curves were fitted to the logistic equation using nonlinear regression (PRISM 5.0; Graph Pad Software Inc, San Diego, California). The mean half-maximal inhibitory concentration (IC50, also expressed as the negative logarithm producing 50% inhibition, log IC50) of reagents was calculated to compare the inhibitory effects of each drug on amplitude of contraction and activity integral (AUC) between patient groups.
Where applicable, data were stratified by gestational age group according to preterm (<37 weeks gestation) or term delivery (>37 weeks gestation). All data were analyzed statis- tically with Prism 5.0. Mann-Whitney U tests were used for nonparametric data and unpaired t test for parametric data. A chi-square test for categorical data was used where appropriate. The logIC50 values were compared on the basis of the F test for the extra sum of squares principle. Unless stated otherwise, all values represent the mean + standard error of the mean (SEM) where ‘‘n’’ is the number of samples and each repre- senting a different woman. P < .05 was taken as level of significance.
Solutions
All chemicals were purchased from Sigma (Poole, Dorset, United Kingdom) unless otherwise stated. Atosiban stock solu- tion (10 mmol/L) and oxytocin stock solution (10 mmol/L) were prepared in deionized water and stored at 20 C. For stability, bovine serum albumen (0.1%, w/v) was added to atosiban stock solution as per manufacturer’s instructions. Progesterone and indomethacin stock solutions (100 mmol/L) were dissolved in absolute ethanol and DMSO, respectively, and stored at room temperature. Appropriate drug dilutions were prepared by direct dilution into PSS made fresh each day.
Results
Spontaneous Contractions
Myometrial strips achieved stable, spontaneous contractions within 2 to 3 hours of superfusion under 2 mN standard stretch. Preliminary studies were performed to assess the time course of
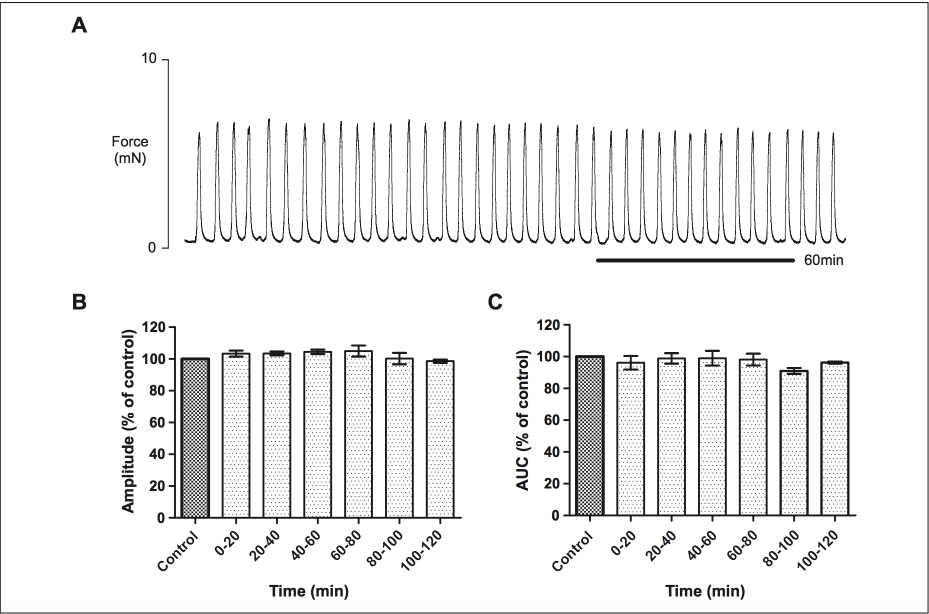
Figure 1. Time course assessment of the stability of spontaneous contractions of human myometrium in vitro. A, Contractions remained stable for over 3 hours of recording without significant decrease in amplitude or area under the curve during the time equivalent of experimental maneuvers (n 1⁄4 5). B and C, show the mean amplitude of contraction and area under the curve (AUC) calculated during each 20-minute period following the initial control, expressed as a percentage of control where control equals 100%.
experiments and tissue contractile stability (n 1⁄4 5). As expected and shown in previous and others studies,22,24,25 spontaneous contractions remained phasic and regular for >3 hours (see Figure 1A). During the equivalent 2-hour time period of drug applications (following control activity), there was no significant change in amplitude of spontaneous contrac- tions or activity integral (Figure 1B and C).
Effect of Progesterone
Progesterone produced a concentration-dependent inhibitory effect on the amplitude of myometrial contractions and activity integral for all myometrial strips examined (n 1⁄4 12 singleton and n 1⁄4 22 twin). Representative recordings demonstrating the effect of progesterone and vehicle on spontaneous contractions and concentration–response curves are shown in Figure 2A-C. As can be seen, there is little or no effect of vehicle (n 1⁄4 6) until used at concentrations greater than the respective highest con- centration of progesterone examined.
The IC50s for amplitude of spontaneous contractions under pro- gesterone in singleton and twin myometrium were 4.39 10 5 mol/L (95% confidence interval [CI]: 2.98-6.45 10 5 mol/L) and 3.37 10 5 mol/L (95% CI: 2.79-4.08 10 5 mol/L), respectively. Statistical comparison of the log IC50 values found no significant difference between groups (P 1⁄4 .172). The IC50s for AUC were 2.21 10 5 mol/L (95% CI: 2.93 10 6 to 1.16 10 4 mol/L) and 1.19 10 5 mol/L (95% CI: 6.36 10 6 to 2.21 10 5 mol/L) for singleton and twin myometrium, respectively. Similarly the logIC50 values for activity integral were not significantly different between groups (P 1⁄4 .511).
To account for gestational age differences between single- ton and twin pregnancy groups, the effects of progesterone only in those samples taken from women at term (>37 weeks gesta- tion, see Table 1B for sample demographics) were compared. We found no significant difference between logIC50 values achieved by progesterone for amplitude or AUC in myo- metrium from singletons and twins at term (P 1⁄4 .281 and P 1⁄4 .328, respectively, Figure 2D and E).
The twin pregnancy group was further stratified according to delivery at term or preterm. In this cohort, the median length of gestation for the preterm women with twins was 253 days (interquartile range 226-257), which as expected, was signifi- cantly shorter than the term twin group (P < .001) and mean
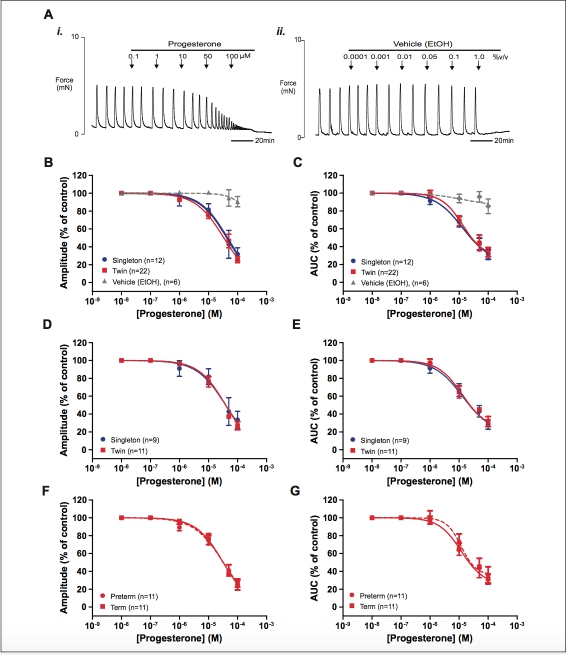
Figure 2. The effect of progesterone on spontaneous myometrial contraction. (A) Representative recordings (paired strips) showing (i) the inhibitory effect of increasing concentrations of progesterone on myometrial contraction and (ii) the effect of vehicle (EtOH). Arrows indicate the points at which progesterone or vehicle was added to the bath. (B and C) show concentration–response curves for the effect of proges- terone on all singleton (blue), twin (red), and appropriate dilution of vehicle (gray, paired strips) on myometrial contraction amplitude and activ- ity integral (AUC), respectively. (D and E) show the same dose–response data for term-only samples and (F and G) show the data from twin myometrium only stratified by gestational age group. Data are presented as mean + standard error of the mean (SEM) percentage of amplitude and AUC before the application of progesterone (control). AUC indicates area under the curve. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
combined birthweight was also significantly increased in the term group (P 1⁄4 .006). However, we found no significant dif- ference in logIC50 values for the effect of progesterone on spontaneous contraction amplitude or AUC between preterm and term-pregnant myometrium in twins (P 1⁄4 .545 and P 1⁄4 .719, respectively, Figure 2F and G).
Effect of Indomethacin
Indomethacin produced a concentration-dependent inhibition on both the amplitude of myometrial contraction and AUC for all samples (n 1⁄4 10 singleton and n 1⁄4 22 twin). Typical record- ings of spontaneous myometrial contractions demonstrating the tocolytic effect of indomethacin in comparison with a time- matched vehicle (DMSO) control are shown in Figure 3Ai and ii. Concentration–response curves are shown in Figure 3B and C. The IC50s for indomethacin on amplitude of spontaneous myometrial contraction from singletons and twins were 4.58 10 5 mol/L (95% CI: 8.06 10 6 to 2.56 10 4 mol/L) and 6.31 10 5 mol/L (95% CI: 5.70 10 6 to 6.98 10 4 mol/L), respectively. However, the logIC50 values were not statistically different (P 1⁄4 .831). Similarly, there was no differ- ence in the logIC50 values for indomethacin on AUC between the 2 groups (P 1⁄4 .727) with IC50 values being 3.54 10 5 mol/L (95% CI: 1.27-9.88 10 5 mol/L) in singletons and 2.88 10 5 mol/L (95% CI: 1.57-5.30 10 5 mol/L) in twins.
Analysis of term-only samples also found no significant dif- ference between logIC50 values achieved by indomethacin for amplitude and AUC in myometrium from singletons and twins (P 1⁄4 .907 and P 1⁄4 .976, respectively, Figure 3D and E). Similarly, there was also no significant difference in logIC50 values observed for the effects of indomethacin on spontaneous contraction amplitude or AUC according to gesta- tional age group (preterm and term pregnant) twin pregnancies (P 1⁄4 .251 and P 1⁄4 .663, respectively, Figure 3F and G).
Oxytocin-Induced Contractions
The concentration of oxytocin used was based upon its ability to produce regular, stabilized phasic contractions within 30 minutes of its application in our experimental model (see Figure 4Ai), rather than tetanic-like activity observed with greater concentrations (see6). These oxytocin-induced contrac- tions also persisted without significant decrease in amplitude or activity integral for many hours and thus enabled changes in contractile activity under different treatments to be measured.
Effect of Atosiban
As expected, there was little or no effect of atosiban on spon- taneous activity (not shown) therefore only the inhibition of oxytocin-induced contractions was examined. Atosiban was applied in rising concentrations between 1 nmol/L and 1 mmol/L and in some cases (n 1⁄4 10) 10 mmol/L was also inves- tigated. Typical recordings of myometrial contractions stimu- lated with oxytocin and demonstrating the tocolytic effect of atosiban in comparison with a paired, time-matched control (oxytocin only) are shown in Figure 4Ai and ii. As 1 mmol/L atosiban did not cause complete inhibition of oxytocin- induced contractions in most samples (24/34 strips and see Figure 4Aii), the effect of 10 mmol/L was also examined. Figure 4B and C depicts the concentration–response curves for the effect of atosiban on amplitude of oxytocin-induced contrac- tion and AUC, respectively. The IC50s for atosiban for contrac- tion amplitude in singletons and twins were 4.08 10 7 mol/L (95% CI: 6.20 10 9 to 2.67 10 5 mol/L) and 3.57 10 7 mol/L (95% CI: 1.11 10 7 to 1.15 10 6 mol/L), respec- tively. However, the values were not statistically different (P 1⁄4 .9968). Similarly, there was no difference in the logIC50 values for atosiban on AUC between the 2 groups (P 1⁄4 .9106) with IC50 values being 8.72 10 8 mol/L (95% CI: 5.84 10 9 to 1.30 10 6 mol/L) in singletons and 7.64 10 8 mol/L (95% CI: 2.30 10 8 to 2.54 10 7 mol/L) in twins.
Examination of the effect of atosiban on term-only samples also showed no significant difference between the logIC50 values for singleton and twin pregnancy groups for both ampli- tude of contraction and AUC (P 1⁄4 .7906 and P 1⁄4 .5793, respectively, Figure 4D and E). There was also no difference between the logIC50 values observed for the effects of atosi- ban on oxytocin-induced contraction amplitude or AUC when stratified by gestational age group (preterm and term pregnant) in the twin pregnancy group alone (P 1⁄4 .418 and P 1⁄4 .333, respectively, Figure 4F and G).
Effect of Progesterone Under Oxytocin Stimulation
A total of 17 myometrial biopsies (n 1⁄4 8 singletons and n 1⁄4 9 twins) were used in the analysis of the tocolytic efficacy of progesterone on oxytocin-induced contractions. Compared to progesterone’s effect on spontaneous contractions, the logIC50 values for progesterone on oxytocin-induced con- traction amplitude were significantly shifted to the right with IC50 values for contraction amplitude under oxytocin being 8.81 10 5 mol/L (95% CI: 8.01-9.65 10 5 mol/L, n 1⁄4 17) compared to 3.69 10 5 mol/L (95% CI: 3.02-4.50 10 5 mol/L, n 1⁄4 34) for spontaneous contraction amplitude (P < .0001, Figure 5Ai). Similarly, the IC50 values for AUC were also significantly shifted to the right under oxytocin: 7.10 10 5 mol/L (95% CI: 4.83 10 5 mol/L to 1.04 10 4 mol/L, n 1⁄4 17) compared to 3.46 10 5 mol/L (95% CI: 2.65-4.50 10 5 mol/L, n 1⁄4 34) for spontaneous contrac- tions (P 1⁄4 .0017, Figure 5Aii). When stratified according to pregnancy group, that is, singletons and twins, the logIC50 values for activity integral were significantly shifted to the right for both groups (P 1⁄4 .038 singletons and P 1⁄4 .0009 twins). Amplitude of contraction was also significantly shifted to the right for both pregnancy groups (P 1⁄4 .0071 singletons and P < .0001, twins).
Typical recordings of the effect of progesterone on contrac- tions under oxytocin in singletons and twins are shown in Figure 5Bi and ii, respectively, and the associated concentra- tion–response curves for mean amplitude of contraction and
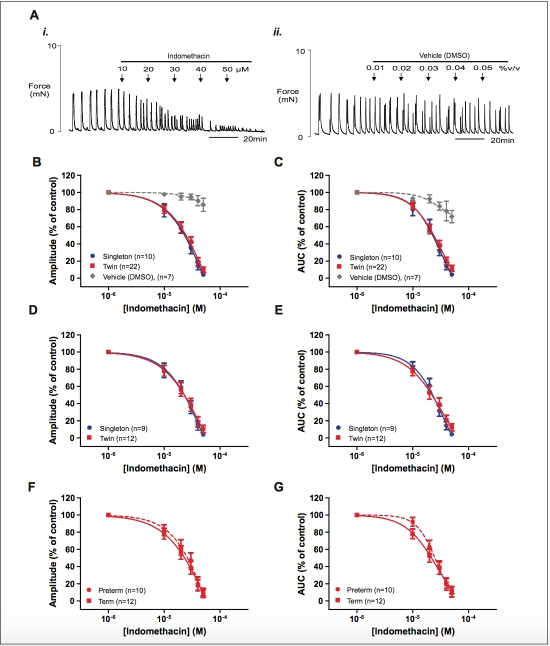
Figure 3. The effect of indomethacin on spontaneous myometrial contraction. (A) Representative recordings (paired strips) showing (i) the inhi- bitory effect of increasing concentrations of indomethacin on myometrial contraction and (ii) the effect of vehicle (dimethyl sulfoxide [DMSO]). (B and C) Concentration–response curves showing the effects of progesterone on singleton (blue), twin (red), and appropriate dilution of vehicle (gray, paired strips) on myometrial contraction amplitude and AUC, respectively. (D and E) show the same data for term-only samples and (F and G) show the data for twin myometrium stratified according to gestational age group. Data are presented as mean + standard error of the mean (SEM) percentage of amplitude and AUC before the application of indomethacin (control). AUC indicates area under the curve. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
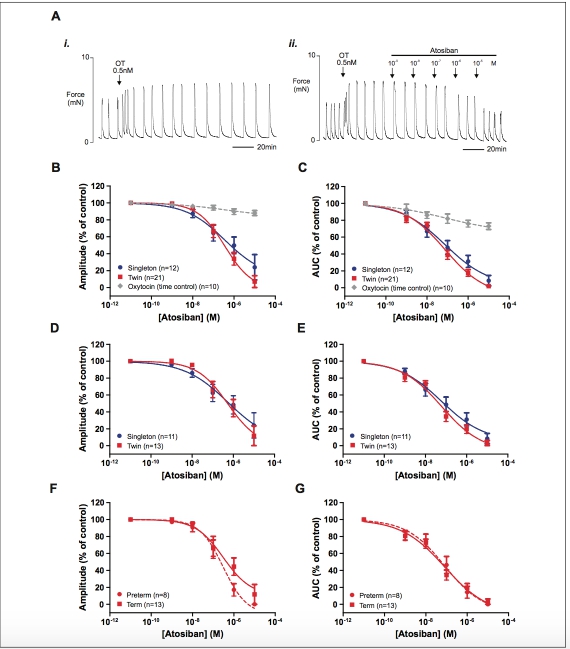
Figure 4. The effect of atosiban on oxytocin-induced (0.5 nmol/L) myometrial contraction. (A) Representative recordings (paired strips) show- ing that (i) contractions induced by 0.5 nmol/L oxytocin persist without significant loss of amplitude for many hours and (ii) the inhibitory effect of increasing concentrations of atosiban on oxytocin-induced myometrial contractions. (B and C) depict the concentration–response curves showing the effects of atosiban on singleton (blue), twin (red), and oxytocin (time control, gray, paired strips) on myometrial contraction ampli- tude and area under the curve (AUC), respectively. (D and E) show the same dose–response data from the term-only groups and (F and G) show the data for the twin myometrium stratified according to gestational age group. Data are presented as mean + standard error of the mean (SEM) percentage of amplitude and activity integral before the application of atosiban (control). (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
activity integral are shown in Figures 4D and 5C. Comparison of the logIC50 values for progesterone’s effect on oxytocin- induced amplitude of contraction and activity integral between twins and singletons found them to be significantly greater in the twin pregnancy group compared to the singleton group (ampli- tude logIC50 1⁄4 4.12 + 0.03 singleton vs 4.01 + 0.03 twins,
P 1⁄4 .0134, AUC logIC50 1⁄4 4.30 + 0.07 singletons vs 3.97 + 0.17 twins, P 1⁄4 .0258, Figure 5C and D), suggesting a greater concentration of progesterone is required to decrease contrac- tions under oxytocin in twins compared to singletons.
Effect of Indomethacin Under Oxytocin Stimulation
For contractions under oxytocin, indomethacin also exerted a tocolytic effect. Similarly to progesterone, a significant right- ward shift in the concentration–response curves was observed under oxytocin (n 1⁄4 17) compared to spontaneous contractions (n 1⁄4 32, singleton and twin data combined). The concentra- tion–response curves for the effect of indomethacin on oxytocin-induced contraction in comparison to spontaneous contractions are shown in Figure 6Ai (amplitude) and Figure 6Aii (AUC). The associated IC50 values for mean amplitude of contraction shifted from 2.48 10 5 mol/L (95% CI: 2.16-2.84 10 5 mol/L) to 5.76 10 5 mol/L (95% CI: 3.65-9.10 10 5 mol/L) under oxytocin (P < .0001) and the IC50 values for AUC increased from 2.40 10 5 mol/L (95% CI: 2.14-2.69 10 5 mol/L) to 6.45 10 5 mol/L (95% CI: 2.49 10 5 mol/L to 1.67 10 4 mol/L) under oxytocin (P < .0001).
Similarly when stratified according to singleton or twin pregnancy group, the logIC50 values for mean contraction amplitude and activity integral were significantly shifted to the right for both groups (P < .01). Figure 6Bi and ii shows repre- sentative recordings of contractions induced by oxytocin in sin- gleton and twin myometrium and the effects of increasing concentration of indomethacin are shown. The logIC50 values for the effect of indomethacin on oxytocin-induced amplitude of contraction were significantly greater in twin myometrium compared to singleton myometrium ( logIC50 1⁄4 4.40 + 0.02 singletons vs 4.20 + 0.02 twins, P < .0001, Figure 6C) and the logIC50 values for activity integral were also signif- icantly greater in twin myometrium compared to singleton myometrium ( logIC50 1⁄4 4.48 + 0.03 singletons vs 4.29 + 0.04 twins, P 1⁄4 .0002, Figure 6D), suggesting that, similarly to progesterone, a greater concentration of indomethacin is required to inhibit contractions induced by oxytocin in twins.
Discussion
Little is known about the myometrium of twin gestations and its responsiveness (or not) to tocolytics as their activity has not been studied extensively in vitro or in vivo. This is the first study to examine the potencies of different tocolytics and the steroid hor- mone progesterone in myometrium from twin pregnancy and is the first to draw direct comparisons with singleton myometrium. We chose to examine atosiban and indomethacin as these are 2 of the most widely used tocolytics (at least in singleton preg- nancy). In addition, although not used acutely as a tocolytic in preterm labor per se, we chose to also examine the effect of an acute application of progesterone on myometrial activity, fol- lowing the differences noted in its prophylactic ability in pre- venting preterm labor in high-risk women with singleton, but not twin pregnancy. We firstly examined the responses to each agent on spontaneously contracting strips of myometrium and then in the presence of a physiological concentration (0.5 nmol/L) of oxytocin to mimic the hormonal milieu in labor.
We find no significant difference between singleton and twin myometrium in the logIC50 values for indomethacin or progesterone on spontaneous activity or atosiban on oxytocin-induced contractions, either when samples of all gestations were combined or when gestationally age controlled for (by way of grouping < or 37 weeks). In addition, we find no difference in logIC50 values between twins when grouped according to gestation, that is, preterm versus term. Data there- fore suggest that on spontaneous contractions, indomethacin and progesterone are as potent in suppressing myometrial contractions in twin and singleton pregnancies; similarly for atosiban, it is as potent in suppressing oxytocin-induced con- tractions in twins as it is in singletons.
However under oxytocin stimulation, we find that the response to progesterone and indomethacin is significantly shifted to the right, showing a greater concentration is required to overcome the effects of oxytocin’s drive on contraction. This was true for both singleton and twin myometrium. Comparison of the potency of these agents between the 2 groups, however, found that under the influence of oxytocin, a greater concentra- tion of progesterone or indomethacin is required to inhibit con- tractions by half in twins compared to singletons under the same experimental conditions. Our previous data showed that twins have a greater response to oxytocin,6 suggesting twin myometrium is possibly more sensitive to oxytocin, which may indicate differences in receptor number. Thus it is plausible that, should oxytocin be playing a role in preterm labor, the heightened contractile response to oxytocin observed in twins as previously reported6 would necessitate a greater concentra- tion of suppressant to overcome this. In trials of prophylactic progesterone for prevention of preterm delivery, progesterone administered antenatally was not found to reduce the risk of delivering preterm in twins,10,14-18 however, it does in a select group of singleton women at high risk.9-13 The doses used were largely comparable across trials but there were differences in the type of progesterone and mode of administration used.
It is difficult to directly compare the concentration of serum/ plasma/blood oxytocin found in laboring women with what the effective concentration around myocytes in the uterus will be, and in any case, receptor number rather than concentration is considered to be the important regulatory mechanism.26,27 We have therefore used a concentration of oxytocin in vitro that simulates the clinical effects of oxytocin, that is, contractions are strengthened but remain phasic (and are therefore also suit- able for concentration–response experiments requiring multi- ple applications of reagent) but is at the lower end of values found in other studies (eg28,29) and including our previous study.6 The effects of oxytocin on agent potency observed, par- ticularly in twins, may therefore be an underestimate of its effect in vivo, increasing the significance of our findings. How- ever, higher concentrations of oxytocin such as previously used by us and others were not studied here and this is a limitation of
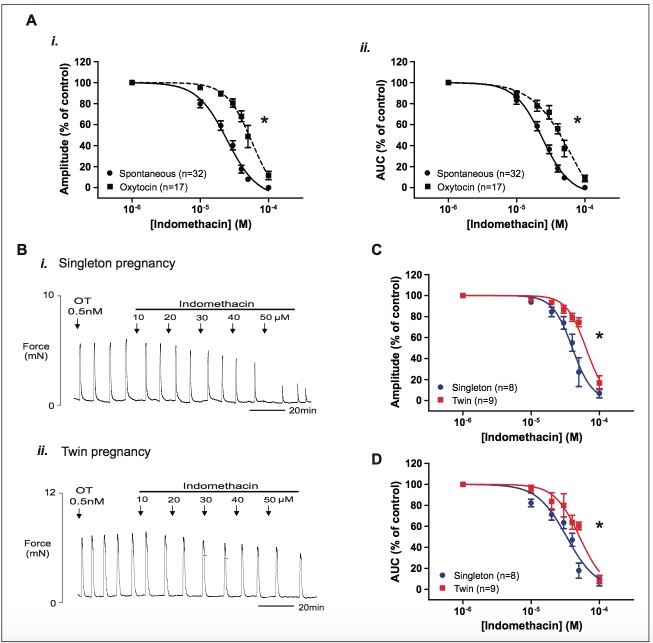
Figure 6. The effect of indomethacin on oxytocin-induced myometrial contraction. Increasing concentrations of indomethacin were applied to strips of myometrium contracting under stimulation with oxytocin (0.5 nmol/L) from women with singleton (n 1⁄4 8) or twin (n 1⁄4 9) pregnancy. (A) Concentration–response curves showing the effect of indomethacin on spontaneous (circles) and oxytocin-induced (squares) myometrial contraction amplitude (i) and activity integral (ii, all data combined). Note that oxytocin caused a significant rightward shift in dose–response curves to indomethacin. (B) Representative recordings showing the inhibitory effect of increasing concentrations of indomethacin on oxytocin- induced myometrial contractions in (i) singleton and (ii) twin myometrium. (C and D) show concentration–response curves for the effects of indomethacin on singleton (blue) and twin (red) oxytocin-induced myometrial contraction amplitude and area under the curve (AUC) derived from experiments shown in (Bi) and (ii). Data are presented as mean + standard error of the mean (SEM) percentage of amplitude and activity integral before the application of progesterone (control).*P < .001 indicates a significant difference between the logIC50 values. IC50 indicates half-maximal inhibitory concentration. (The color version of this figure is available in the online version at http://rs.sagepub.com/.)
this study. We did not, however, find a difference in the potency of atosiban in inhibiting oxytocin-induced myometrial contractions between groups. The response to atosiban observed is however consistent with that of a competitive oxytocin recep- tor antagonist.
The acute application of both indomethacin and progester- one (albeit at high concentrations) clearly suppresses myome- trial contractions in vitro. The rapid action of progesterone suggests it is acting through a nongenomic mechanism, which is thought to be mediated by nongenomic progesterone recep- tors residing on the cell membrane, for example, cell mem- brane progesterone receptors a, b, and g.30,31 The nature of these receptors and the mechanism through which they act to inhibit contraction, however, are still not fully elucidated but potassium channels are not thought to be involved.25 Progester- one’s use clinically is mainly prophylactic, that is, it is given antenatally to prevent preterm labor onset rather than as an acute tocolytic to inhibit preterm labor contractions. The dose required to suppress myometrial contractions by acute applica- tion in vitro as shown in this study (and others, eg25), however, is not likely to be achieved with doses administered clinically. Progesterone has been trialled as an acute tocolytic in a few studies including a recent trial of preterm labor patients (200 mg daily vaginal administration following 48-hour acute tocolysis), however, it was not found to be any better than pla- cebo in decreasing preterm birth.32
Indomethacin is known predominately for its effects as an inhibitor of COX and therefore prostaglandin synthesis. Its rapid action in abolishing myometrial contractions however also suggests a mode of action other than through inhibiting prostaglandin production, which is further supported by the fact that the potency for indomethacin to suppress myometrial con- tractions found in this study and others33,34 is much higher than that reported to inhibit COX.35 Its acute effects on inhibition of myometrial contractions are thought to be mediated in part by inhibition of Ca currents34 and thus reduced Ca entry and avail- ability for contraction. This would, in part, explain the large rightward shift observed in the IC50s for indomethacin under oxytocin, particularly given that one of oxytocin’s many actions on the myometrium includes increasing intracellular Ca levels, including increasing Ca entry.26 The therapeutic dose of indomethacin given however is much lower than that used in this study to inhibit contractions: little or no effect on myometrial contraction was observed at concentrations <10 mmol/L, which is more likely to be within the therapeutic range. Whether indomethacin’s tocolytic effect in vivo is via inhibition or blocking of Ca currents is therefore not known.
Limitations
A preterm, singleton arm to this study would have added value and is therefore its absence is a limitation of this study. The main reason for the absence of this group was that most single- ton preterm women deliver vaginally and therefore we are unable to collect a biopsy. While gestational age may be a con- tributing factor, we attempted to address this by examining the effect of the different agents in gestationally age-matched sam- ples. However, this could only be done according to an arbi- trary cuff-off point of 37 weeks, that is, the definition of preterm delivery. When stratified according to gestational age group (preterm: <37 weeks or term: 37 weeks), our term only twin group still had a lower mean gestational age compared to our term singleton group. This, however, is not surprising given that rates of perinatal mortality and morbidity are increased in women whose twin pregnancy continues beyond 37 weeks36-38 and findings from a randomized control trial support recom- mendations for women with an uncomplicated twin pregnancy to deliver before 38 weeks gestation.39 At LWH, it is our prac- tice to offer delivery from 37 weeks in dichorionic and from 36 weeks in monochorionic twins, as per NICE guidelines.40 Thus many obstetricians will opt to deliver twins electively between 36 and 38 weeks. In fact delivery of twin pregnancy at 38 weeks or greater is considered by some to be the equiva- lent of ‘‘postterm’’ in singletons.41 However within the twin pregnancy group, we do not find any differences in the response to these agents on spontaneous contractions when comparing preterm versus term pregnancy groups. We did not however examine women in labor. Additionally, the parity of women in the twin cohort was lower than the singleton cohort, that is, a higher number of twin samples were from nulliparous mothers, which could not be controlled for. Thus the significant differences we find could also be influenced parity as well as gestation.
A possible limitation to our data is setting the optimal stretch on the myometrial samples. Our approach was to per- form the experiments under experimental conditions that gave the same amount of stretch to all samples. This resulted in strong, consistent phasic contractions in all samples used for analysis, as shown throughout our records. It could perhaps be argued that this does not optimally reflect the situation in vivo, that is, twin myometrium is likely to be subjected to a greater degree of stretch, as is perhaps evident by the higher birthweight in the twin arms of this study. In the light of the limited data on this, as well as the difficulty of extrapolating to in vivo data and the benefit of not introducing another vari- able into our comparisons, we chose to unify the stretch across samples. Further work investigating the effect of tocolytic and progesterone potencies under different experimental conditions including stretch would be useful.
In summary, this study has examined the potencies of acute application of 2 known tocolytics, atosiban, and indomethacin and of the steroid hormone progesterone on both spontaneous and oxytocin-induced myometrial contractions from singleton and twin pregnancies. Our findings indicate that in standard in vitro conditions and in the absence of oxytocin stimulation, the myometrium from twin pregnancies is as responsive to indomethacin and progesterone as the singleton myometrium. However, under low-dose stimulation with oxytocin, the effect of both these agents is lessened and a greater concentration of both drugs is required to inhibit oxytocin-induced myome- trial contraction in twins compared to singletons. These pres- ent data, together with our previous findings demonstrating a heightened response to oxytocin in vitro in twin pregnancy,6 suggest that if myometrial oxytocin receptor expression and receptor signaling is heightened in twins, the clinical efficacy of treatment in multiple gestation may be reduced. Although treatment with tocolytic agents such as indomethacin is rarely used in twin pregnancy and progesterone is also contraindi- cated in multiple pregnancies, this study highlights important pharmacological differences between singleton and twin myo- metrium, which will further help delineate the underlying phy- siological differences between these 2 pregnancy groups. Future studies will seek to examine the potency of tocolytics in combination with oxytocin receptor antagonists with the aim of targeting multiple pathways involved in myometrial contraction in preterm labor.
Authors’ Note
All work was performed in the Department of Cellular and Molecular Physiology and The Harris-Wellbeing Preterm Birth Centre, Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom. Some of these data were presented as a poster at 61st Annual Society for Reproductive Investigation meeting, Florence, Italy, March 26-29, 2014 and given as an oral presentation at the 62nd Annual Society of Reproductive Investigation Myometrial Satel- lite Symposium, San Francisco, California, USA, March 25, 2015. The funders had no involvement in the study design, collection, anal- ysis or interpretation of data, the writing of the report, or in the deci- sion to submit the article for publication.
Acknowledgments
We thank Sparks, the children’s charity for funding this research. We also thank the patients and staff at Liverpool Women’s Hospital for assisting in the collection of biopsies.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Sparks, United Kingdom.
References
-
March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization; 2012.
-
Martin JA, Hamilton BE, Osterman MJ. Three decades of twin births in the United States, 1980-2009. NCHS Data Brief. 2012; (80):1-8.
-
Stock S, Norman J. Preterm and term labour in multiple pregnan- cies. Semin Fetal Neonat Med. 2010;15(6):336-341.
-
Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760-765.
-
Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturi- tion syndrome. BJOG. 2006;113(suppl 3):17-42.
Turton P, Arrowsmith S, Prescott J, et al. A comparison of the contractile properties of myometrium from singleton and twin pregnancies. PLoS One. 2013;8(5):e63800.
Gyetvai K, Hannah ME, Hodnett ED, Ohlsson A. Tocolytics for preterm labor: a systematic review. Obstet Gynecol. 1999;94(5 pt 2):869-877.
Yamasmit W, Chaithongwongwatthana S, Tolosa JE, Limpongsa- nurak S, Pereira L, Lumbiganon P. Prophylactic oral betami- metics for reducing preterm birth in women with a twin pregnancy. Cochrane Database Syst Rev. 2012;9:Cd004733.
Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent pre- term delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Proges- terone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462-469.
HassanSS,RomeroR,VidyadhariD,etal.Vaginalprogesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011; 38(1):18-31.
Rai P, Rajaram S, Goel N, Ayalur Gopalakrishnan R, Agarwal R, Mehta S. Oral micronized progesterone for prevention of preterm birth. Int J Gynaecol Obstet. 2009;104(1):40-43.
DeFrancoEA,O’BrienJM,AdairCD,etal.Vaginalprogesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a sec- ondary analysis from a randomized, double-blind, placebo- controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):697-705.
Rouse DJ, Caritis SN, Peaceman AM, et al. A trial of 17 alpha- hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357(5):454-461.
NormanJE,MackenzieF,OwenP,etal.Progesteroneforthepre- vention of preterm birth in twin pregnancy (STOPPIT): a rando- mised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373(9680):2034-2040.
Combs CA, Garite T, Maurel K, Das A, Porto M. 17-hydroxyprogesterone caproate for twin pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol. 2011;204(3):221. e221-e228.
Rode L, Klein K, Nicolaides KH, Krampl-Bettelheim E, Tabor A. Prevention of preterm delivery in twin gestations (PREDICT): a multicenter, randomized, placebo-controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet Gynecol. 2011;38(3):272-280.
Briery CM, Veillon EW, Klauser CK, et al. Progesterone does not prevent preterm births in women with twins. South Med J. 2009; 102(9):900-904.
Caritis SN, Rouse DJ, Peaceman AM, et al. Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Obstet Gynecol. 2009;113(2 pt 1): 285-292.
Combs CA, Garite T, Maurel K, Das A, Porto M. Failure of 17-hydroxyprogesterone to reduce neonatal morbidity or prolong triplet pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol. 2010;203(3):248. e241-e249.
Luckas MJ, Wray S. A comparison of the contractile properties of human myometrium obtained from the upper and lower uterine segments. BJOG. 2000;107(10):1309-1311.
-
Arrowsmith S, Quenby S, Weeks A, Burdyga T, Wray S. Poor spontaneous and oxytocin-stimulated contractility in human myo- metrium from postdates pregnancies. PLoS One. 2012;7(5): e36787.
-
Shmigol AV, Eisner DA, Wray S. Properties of voltage-activated [Ca2þ]i transients in single smooth muscle cells isolated from pregnant rat uterus. J Physiol. 1998;511(pt 3):803-811.
-
Zhang J, Kendrick A, Quenby S, Wray S. Contractility and cal- cium signaling of human myometrium are profoundly affected by cholesterol manipulation: implications for labor? Reprod Sci. 2007;14(5):456-466.
-
Anderson L, Martin W, Higgins C, Nelson SM, Norman JE. The effect of progesterone on myometrial contractility, potassium chan- nels, and tocolytic efficacy. Reprod Sci. 2009;16(11):1052-1061.
-
Arrowsmith S, Wray S. Oxytocin: its mechanism of action and receptor signalling in the myometrium. J Neuroendocrinol. 2014;26(6):356-369.
-
Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150(6):734-741.
-
Balki M, Erik-Soussi M, Kingdom J, Carvalho JC. Oxytocin pre- treatment attenuates oxytocin-induced contractions in human myometrium in vitro. Anesthesiology. 2013;119(3):552-561.
-
Gullam JE, Blanks AM, Thornton S, Shmygol A. Phase-plot anal- ysis of the oxytocin effect on human myometrial contractility. Eur J Obstet Gynecol Reprod Biol. 2009;144(suppl 1):S20-S24.
-
Falkenstein E, Heck M, Gerdes D, et al. Specific progesterone bind- ing to a membrane protein and related nongenomic effects on Ca2þ- fluxes in sperm. Endocrinology. 1999;140(12):5999-6002.
-
Karteris E, Zervou S, Pang Y, et al. Progesterone signaling in human myometrium through two novel membrane G protein- coupled receptors: potential role in functional progesterone with- drawal at term. Mol Endocrinol. 2006;20(7):1519-1534.
Martinez de Tejada B, Karolinski A, Ocampo MC, et al. Preven- tion of preterm delivery with vaginal progesterone in women with preterm labour (4P): randomised double-blind placebo-controlled trial. BJOG. 2015;122(1):80-91.
Vane JR, Williams KI. The contribution of prostaglandin produc- tion to contractions of the isolated uterus of the rat. Br J Pharma- col. 1973;48(4):629-639.
Sawdy R, Knock GA, Bennett PR, Poston L, Aaronson PI. Effect of nimesulide and indomethacin on contractility and the Ca2þ channel current in myometrial smooth muscle from pregnant women. Br J Pharmacol. 1998;125(6):1212-1217.
Riendeau D, Percival MD, Boyce S, et al. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br J Pharmacol. 1997; 121(1):105-117.
Cheung YB, Yip P, Karlberg J. Mortality of twins and singletons by gestational age: a varying-coefficient approach. Am J Epide- miol. 2000;152(12):1107-1116.
Hartley RS, Emanuel I, Hitti J. Perinatal mortality and neonatal morbidity rates among twin pairs at different gestational ages: optimal delivery timing at 37 to 38 weeks’ gestation. Am J Obstet Gynecol. 2001;184(3):451-458.
Luke B, Minogue J, Witter FR, Keith LG, Johnson TR. The ideal twin pregnancy: patterns of weight gain, discordancy, and length of gestation. Am J Obstet Gynecol. 1993;169(3): 588-597.
Dodd JM, Crowther CA, Haslam RR, Robinson JS. Elective birth at 37 weeks of gestation versus standard care for women with an uncomplicated twin pregnancy at term: the Twins Timing of Birth Randomised Trial. BJOG. 2012;119(8):964-973.
NICE. National Institute for Health and Care Excellence Clinical Guidelines: Multiple pregnancy: The management of twin and tri- plet pregnancy in the antenatal period (CG129). London: NICE; 2011.
Minakami H, Sato I. Reestimating date of delivery in multifetal pregnancies. JAMA. 1996;275(18):1432-1434.
