Carbetocin versus oxytocin after caesarean section: similar e cacy but reduced pain perception in women with high risk of postpartum haemorrhage
Maria De Bonis1, Michela Torricelli1, Licia Leoni2, Paolo Berti2, Valentina Ciani1, Rosa Puzzutiello2, Filiberto Maria Severi1 & Felice Petraglia1
1Section of Obstetrics and Gynecology, Department of Pediatrics, Obstetrics, and Reproductive Medicine, University of Siena, Siena, Italy and 2Department of Anesthesia and Intensive Care, AUOS-University of Siena, Siena, Italy
Objective: To compare the e ectiveness of carbetocin with oxytocin with respect to maintain adequate uterine tone and to reduce the incidence and severity of postpartum haemorrhage. Moreover safety, adverse e ects and the need of additional medications were evaluated. Methods: Prospective controlled clinical trial. We compared the e ect of a single dose of carbeto- cin (n = 55) with oxytocin infusion (n = 55) in a women popula- tion undergoing to elective caesarean section with regional subarachnoid anaesthesia with at least one risk factor for post- partum haemorrhage. Results: The mean ± SD of postoperative pain in the day of surgery in carbetocin group was signi cantly lower than in oxytocin group and remained signi cant till the third day after caesarean section. In the day of surgery and the rst day after surgery, women of carbetocin group who needed analgesic drugs were signi cantly lower than women of oxytocin group. The di erences of diuresis and of diuretic drugs need were not statistically signi cant between the two groups. Con- clusions: A single carbetocin injection is e cacious and safe on the maintenance of uterine tone and on the limitation of blood losses, in peri- and in postoperative period. In addition, carbe- tocin was able to reduce pain perception during postoperative days improving quality life of women.
Keywords: Carbetocin, diuresis; oxytocin, pain, postpartum haemorrhage, safety pro le
Introduction
Postpartum haemorrhage (PPH) is de ned as blood loss of at least 500 ml a er vaginal delivery and 1000 ml a er caesarean sec- tion (CS) and/or the need for a blood transfusion within 24 h of delivery [1,2]. It occurs in 5% of all deliveries [2,3] and represents the most important cause of maternal morbidity and mortality worldwide [3–6]. e risk of PPH is much higher for women un- dergoing CS [7]. Uterine atony is the rst cause of haemorrhage at the time of delivery, followed by genital tract injury and retained placenta necessitating manual removal [1,8]. Risk factors for PPH, such as grande multiparity, multiple pregnancy, polyhydramnios, foetal macrosomia, uterine miomas, placenta praevia, prolonged labour, chorioamnionitis and previous PPH, have remained rela- tively constant [1,2].
Despite evidence that active management of the third stage of labour reduces the incidence of PPH, expectant or physiological management are still widely practised. Factors accounting for this situation include the desire for a more natural experience of childbirth, the philosophy that active management is unnecessary in low-risk women, and avoid- ance of the adverse effects of conventional uterotonic agents [6,9–11]. In contrast, active management involves the clinician intervening in the process through three interrelated but in- dependent processes: (1) the administration of a prophylactic uterotonic (oxytocic) drug; (2) cutting and clamping the cord shortly after birth of the infant and (3) controlled traction of the umbilical cord [2,4,6].
Current strategies for preventing PPH include the prophylactic use of uterotonic agents to enhance natural uterine contraction and retraction following CS and in the third stage of labour for vaginal delivery [2,4,6]. Oxytocin is the most widely used utero- tonic agent [6,7], but it has only a half-life of 4–10 min [12] and must be administered as a continuous intravenous infusion to achieve sustained uterotonic activity.
Recent interest has focused on the prophylactic use of the oxy- tocin receptor agonist carbetocin [12–16].
Carbetocin is a long-acting synthetic analogue of oxytocin with uterotonic activity resulting from its binding to oxytocin recep- tors on the myometral cells [17,18] and it is currently approved in 23 countries for prevention of uterine atony and excessive bleed- ing following caesarean delivery in spinal or epidural anaesthesia [12]. It can be administered as a single-dose injection, either in- travenously or intramuscularly [19]. Intravenously administered carbetocin has a half-life of ~40min, around 4–10 times longer than that reported for oxytocin [20]. Adverse events reported by at least 10% of women who received prophylactic intravenous carbetocin following caesarean delivery were headache, tremor, hypotension, ushing, nausea, abdominal pain, itching and feel- ing of warmth [12]. Uncommon or sporadic adverse events were tachycardia, sweating, dizziness, chest pain, vomiting, metallic taste [12].
A case–control study involving pregnant women at risk for PPH was conducted to compare the e ectiveness of a single intravenous (i.v.) injection of carbetocin with that of oxytocin i.v. infusion with respect to intraoperative blood loss, ability to maintain adequate uterine tone and to reduce the incidence and severity of PPH. e two treatments were also compared for safety and adverse e ects, and for the need of additional medications.
Methods
is was a prospective controlled clinical trial conducted between 1 June 2009 and 31 May 2010. We consecutively enrolled at the Department of Pediatrics, Obstetrics, and Reproductive Medi- cine, University of Siena, Siena, Italy a group of pregnant women (n = 110) undergoing to CS with at least one risk factor for PPH. e patients were divided in two groups (n = 55) with blinding to the study medication.
Only term pregnancies (a er 37 weeks of gestation) with at least one risk factor of PPH (grande multiparity, multiple preg- nancy, previous CS, polyhydramnios, foetal macrosomia, uterine miomas, placenta praevia, prolonged labour, chorioamnionitis and previous PPH) were included [8]. In particular, among women receiving carbetocin are included those with (i) previ- ous CS (n=45); (ii) multiple pregnancy (n=7); (iii) previous surgery for uterine miomas (n=2) and (iv) polyhydramnios (n = 1). Moreover, among those receiving oxytocin there are women with: (i) previous CS (n=43); (ii) multiple pregnancy (n=8); (iii) previous surgery for uterine miomas (n=2); (iv) polyhydramnios (n = 1) and (v) foetal macrosomia (n = 1). Preec- lampsia, eclampsia, cardiovascular, kidney or hepatic diseases and epilepsy were considered contraindications to the treatment with carbetocin. Patients at risk of PPH undergoing to vaginal delivery were excluded from the study. Women undergoing CS with general anaesthesia were also excluded, because carbetocin is licensed for use with regional anaesthesia only. Furthermore, we excluded women having emergency CS for foetal or maternal distress where, due to time constraints, it was not possible and/ or appropriate to recruit.
All women underwent to elective CS with regional subarach- noid anaesthesia with bupivacaine 0.5% (10mg) and fentanyl (25 μg).
Each women had a starter intraoperative dose of analgesia with i.v. buprenorphine (0.3mg diluted in 100ml of saline solution) and paracetamol (1 g i.v.) administered in 15 min at the end of CS. Postsurgery analgesia was set with i.v. elastomeric pump for 30 h containing buprenorphine 0.9 mg. e analgesic rescue dose was paracetamol (1 g i.v.) administered in 15 min (maximum dose 1 g every 6 h).
We compared the e ect of a single dose (100 μg i.v.) of car- betocin with oxytocin (10 international units (IU) i.v. followed by 20 IU i.v. infusion in 24 h. e study medication (carbetocin or oxytocin) was diluted in 10ml normal saline and adminis- tered slowly (over 30–60 s) intravenously by the anaesthetist a er the birth of the baby before placental delivery. e slow administration has been shown to reduce the potentially harm- ful haemodynamic e ects of oxytocin [20,21] (and presumably carbetocin).
e following intra- and postoperative parameters were evalu- ated: vital signs, uterine involution and amount of lochia, serum haemoglobin, postoperative pain and need of analgesic drugs, diuresis and need diuretic drugs and adverse e ects.
Vital signs (blood pressure and heart rate) were evaluated dur- ing CS and 30, 60 and 120min, 6 and 12h a er CS and twice a day the rst, second and third day a er surgery.
Lochia, evaluated by visual estimation of blood loss [14], uter- ine involution and tone were assessed during CS and 30, 60, 90 and 120 min, 6 and 12 h a er CS and twice a day the rst, second and third day a er surgery.
Serum haemoglobin levels were documented by comparing the maternal haemoglobin concentration on admission to hospi- tal with that measured 24 h a er delivery.
Postoperative pain and the need of analgesic drugs were evalu- ated in Day 0 (day of surgery), Days 1, 2 and 3 a er CS. e perceived pain was evaluated by a Visual Analogic Scale (VAS) that visually represents the magnitude of pain warned by patients. e amplitude is represented by a line, usually 10cm long, in which one end indicates the absence of pain and the other represents the worst pain imaginable. e scale, which contains from 0 to 10, is completed by the patient, who is asked to draw a line on the sign representing the level of pain experienced. We considered a value ≥4 the minimum score for administration of the rescue dose analgesic drugs.
Diuresis was measured by urinary catheter for 24h a er sur- gery and was estimated every 4 h the day of CS. Moreover, diuresis was estimated every 6h the rst day and twice a day during the second and the third day a er surgery.
e diuretic drug that we administered in case of oliguria is de ned as a urine output <1 ml/kg/h (furosemide 25 mg i.v.).
Statistical analysis
Quantitative variables of data were described as means ± stan- dard deviation. Comparison between two group variables was performed by Kruskal–Wallis one-way analysis, followed by a post-hoc Dunn’s test when the data were not normally distrib- uted. Simple group comparison was made using chi-square tests for categorical variables. Statistical analysis was performed using the GraphPad Prism version 5.00 for Windows (GraphPad So - ware, Inc., San Diego, CA). Statistical signi cance was assumed whenever p < 0.05.
Results
e main clinical and demographic characteristics of the study population are summarized in Table I.
Regarding the following parameters: intraoperative blood loss, uterine involution and tone, amount of lochia, vital signs and se- rum haemoglobin and adverse e ects, there was not statistically signi cant di erence between carbetocin and oxytocin group. In particular, intraoperative blood loss was >2000 ml in one case of twin pregnancy and in one case of triplet pregnancy in study and control group, respectively; however, there was no need for blood transfusion.
Although in only one case infusion of oxytocin (5 IU i.v.) was necessary as additional uterotonic intervention in the carbetocin group, in eight cases of oxytocin group were further infused with intraoperative oxytocin (5 IU i.v.), in other eight women 10 IU intraoperative oxytocin was necessary, in two cases were infused 15 IU and in one women 20 IU of oxytocin in addition to stan- dard intraoperative dose. In two cases of oxytocin group was also necessary to infuse methilergometrine (0.2 mg).
e di erence between postoperative pain in carbetocin and oxytocin group was statistically signi cant. e mean ± SD of postoperative pain in the day of surgery in carbetocin group (3.98 ± 1.88) was signi cantly lower than in oxytocin group (5.72±1.56) (p<0.001) and remained signi cant till the third day a er CS (carbetocin group 1.91±1.50 and oxytocin group 3.50 ± 1.33) (p < 0.001) (Figure 1).
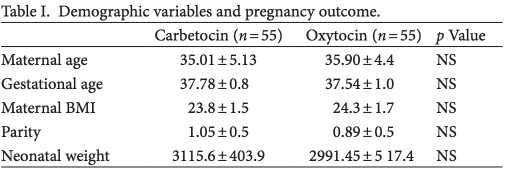

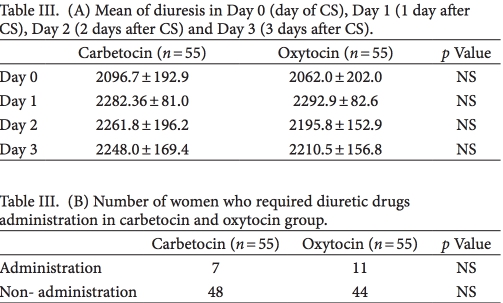
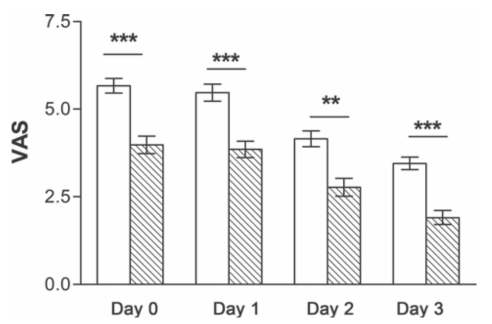
Figure 1. e evaluation of postoperative pain by a Visual Analogic Scale (VAS) in the postoperative days was ***p < 0.001, **p < 0.01.
In the day of CS and in Day 1, women of carbetocin group who needed analgesic drugs were signi cantly lower than women of oxytocin group (p < 0.001) (Table II). e number of women who required analgesic drugs in Days 2 and 3 postoperative period were not statistically signi cant (Table II).
In 11 women of oxytocin group and in seven women of car- betocin group the diuresis of the day of CS was <1ml/kg/h and diuretic drugs were required. e di erences of diuresis and of diuretic drugs need were not statistically signi cant between the two groups as summarized in Table III. None of the women re- quired diuretic drugs during the rst, second and third day a er surgery.
Discussion
Few studies evaluated the e ect of an i.v. injection of carbetocin a er caesarean delivery under regional anaesthesia showing that a single intravenous injection of carbetocin signi cantly reduces the need for additional uterotonic interventions to maintain ade- quate uterine tone and prevent/treat excessive bleeding following caesarean delivery versus intravenous oxytocin [22–24].
e present study con rmed that a single intravenous injec- tion of carbetocin administered during CS signi cantly reduces the need for additional uterotonic interventions in comparison with classic i.v. oxytocin treatment, has the same safety pro le of oxytocin, since vital signs, haematologic values (haemoglobin levels drop) and incidence of adverse e ects were not statistically di erent in the two groups.
Two parameters were studied for the rst time: abdominal pain and diuresis. In none of previous studies, the level of abdominal pain and the use of analgesic drugs a er CS were considered. Our data showed that the level of abdominal pain perceived a er use of carbetocin a er delivery by CS was less than a er use of oxyto- cin. Indeed in carbetocin group small number of women than in oxytocin group had a VAS ≥4. is reduced pain perception was statistically signi cant during all the days of hospitalization a er CS. Moreover, the need of analgesic rescue dose of paracetamol was signi cantly reduced in the carbetocin group. Like oxytocin, carbetocin selectively binds to oxytocin receptors in the smooth muscle of the uterus, stimulates rhythmic contractions, increases the frequency of existing contractions and raises the tone of the uterus musculature [19]. In conclusion, the lower drug quantity administrated and the characteristic of contractions stimulated by carbetocin may explain the lower level of abdominal pain.
The chemical structure of oxytocin resembles that of the an- tidiuretic hormone (vasopressin), so it could have a moderate an- tidiuretic e ect and currently, there were no studies in literature that evaluated the diuresis of women a er administration of car- betocin during CS [19]. It could be associated with hyponatremia and water intoxication a er prolonged administration of high doses, especially when given with a dextrose solution instead of a salt solution [25,26]. Animal studies had shown that carbetocin had some antidiuretic activity (vasopressin activity: <0.025 IU/ ampoule). According to our results, carbetocin had a moder- ate antidiuretic e ect without statistically signi cant di erence in 24h urine output a er surgery between women treated with carbetocin or oxytocin. e use of diuretic drugs was less in the carbetocin group than in the oxytocin group but with no signi - cant di erences, maybe because of the limited study population. is preliminary data showed that carbetocin had a safety pro le comparable with oxytocin but further investigation may be neces- sary to evaluate the amount of antidiuretic e ect of carbetocin.
In conclusion, a single carbetocin injection is e cacious and safe on the maintenance of uterine tone and on the limitation of blood losses, in peri- and in postoperative period, during a deliv- ery by CS. In addition, carbetocin was able to reduce pain percep- tion during postoperative days improving quality life of women, who were able to provide care to newborn a er CS. Moreover, the decrease of postsurgery pain that has been demonstrated a er the use of carbetocin could lead to a reduction in the use of opioid drugs for postsurgery analgesia, thereby reducing side e ects as nausea, vomiting, dizziness, constipation, drowsiness, itching and so on due to the use of this type of drugs.
Acknowledgement
e authors thank the Dr. Giulia Biliotti, Dr. Pamela Dottarelli, Dr. Carlotta Boni and Dr. Daniela Dores for contributing to col- lect data for this study.
Declaration of interest: e authors report no con icts of interest.
References
-
World Health Organization. Recommendations for the Prevention of Postpartum Haemorrhage. Geneva: WHO, 2007.
-
Leduc D, Senikas V, Lalonde AB, Ballerman C, Biringer A, Delaney M, Duperron L, et al. SOGC Clinical Practice Guidelines. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. No. 235 October 2009 (Replaces No. 88, April 2000). Int J Gynecol Obstet 2010;108:258–267.
-
Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol 2008;22:999–1012.
-
Oladapo OT, Akinola OI, Fawole AO, Adeyemi AS, Adegbola O, Loto OM, Fabamwo AO, et al. Nigerian AMTSL Group. Active management of third stage of labor: evidence versus practice. Acta Obstet Gynecol Scand 2009;88:1252–1260.
-
Homer C, Clements V, McDonnell N, Peek M, Sullivan E. Maternal mortality: what can we learn from stories of postpartum haemorrhage? Women Birth 2009;22:97–104.
-
Chong YS, Su LL, Arulkumaran S. Current strategies for the prevention of postpartum haemorrhage in the third stage of labour. Curr Opin Obstet Gynecol 2004;16:143–150.
-
Wedisinghe L, Macleod M, Murphy DJ. Use of oxytocin to prevent haemorrhage at caesarean section–a survey of practice in the United Kingdom. Eur J Obstet Gynecol Reprod Biol 2008;137:27–30.
-
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician– Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol 2006;108:1039–1047.
-
Begley CM, Gyte GM, Murphy DJ, Devane D, McDonald SJ, McGuire W. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev 2010;7:CD007412.
-
Borruto F, Treisser A, Comparetto C. Utilization of carbetocin for prevention of postpartum hemorrhage a er cesarean section: a randomized clinical trial. Arch Gynecol Obstet 2009;280:707–712.
-
Mousa HA, Cording V, Al revic Z. Risk factors and interventions associated with major primary postpartum hemorrhage unresponsive to rst-line conventional therapy. Acta Obstet Gynecol Scand 2008;87:652–661.
-
Rath W. Prevention of postpartum haemorrhage with the oxytocin analogue carbetocin. Eur J Obstet Gynecol Reprod Biol 2009;147:15–20.
-
Boucher M, Nimrod CA, Tawagi GF, Meeker TA, Rennicks White RE, Varin J. Comparison of carbetocin and oxytocin for the prevention of postpartum hemorrhage following vaginal delivery: a double-blind randomized trial. J Obstet Gynaecol Can 2004;26:481–488.
-
Leung SW, Ng PS, Wong WY, Cheung TH. A randomised trial of carbetocin versus syntometrine in the management of the third stage of labour. BJOG 2006;113:1459–1464.
-
Ngan L, Keong W, Martins R. Carbetocin versus a combination of oxytocin and ergometrine in control of postpartum blood loss. Int J Gynaecol Obstet 2007;97:152–153.
-
Su LL, Chong YS, Samuel M. Oxytocin agonists for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2000.
-
Atke A, Vilhardt H. Uterotonic activity and myometrial receptor a nity of Ideamino-l-carba-2-tyrosine (O-methyl)-oxytocin. Acta Endocrinol (Copenh) 1987;19:971–976.
-
Norström A, Andersson A, Vilhardt H. Contractile e ect of oxytocin and Ideamino-l-carba-2-tyrosine (O-methyl)-oxytocin in myometrial tissue from non-pregnant and term pregnant women. Acta Endocrinol (Copenh) 1990;122:566–568.
-
Engstrøm T, Barth T, Melin P, Vilhardt H. Oxytocin receptor binding and uterotonic activity of carbetocin and its metabolites following enzymatic degradation. Eur J Pharmacol 1998;355:203–210.
-
Weis FR Jr, Markello R, Mo B, Bochiechio P. Cardiovascular e ects of oxytocin. Obstet Gynecol 1975;46:211–214.
-
omas JS, Koh SH, Cooper GM. Haemodynamic e ects of oxytocin given as i.v. bolus or infusion on women undergoing caesarean section. Br J Anaesth 2007;98:116–119.
-
Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, Hunt LP, Draycott T. Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double-blind randomised trial. BJOG 2010;117:929–936.
-
Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, Farine D, et al. Double-blind comparison of carbetocin versus oxytocin in prevention of uterine atony a er cesarean section. Am J Obstet Gynecol 1999;180:670–676.
-
Boucher M, Horbay GL, Gri n P, Deschamps Y, Desjardins C, Schulz M, Wassenaar W. Double-blind, randomized comparison of the e ect of carbetocin and oxytocin on intraoperative blood loss and uterine tone of patients undergoing cesarean section. J Perinatol 1998;18:202–207.
-
Hunter DJ, Schulz P, Wassenaar W. E ect of carbetocin, a long-acting oxytocin analog on the postpartum uterus. Clin Pharmacol er 1992;52:60–67.
-
Vercauteren M, Palit S, Soetens F, Jacquemyn Y, Alahuhta S. Anaesthesiological considerations on tocolytic and uterotonic therapy in obstetrics. Acta Anaesthesiol Scand 2009;53:701–709.
