Atosiban versus betamimetics in the treatment of preterm labour in Italy: clinical and economic importance of side-effects
Jaro Wex a,*, Ahmed M. Abou-Setta a,b, Graziano Clerici c, Gian Carlo Di Renzo c
b Department of Paediatrics, University of Alberta, Edmonton, Alberta, Canada
c Department of Obstetrics & Gynecology, University Hospital S. Maria Della Misericordia, Perugia, Italy
ARTICLE INFO
Article history:
Received 6 January 2011
Received in revised form 9 March 2011 Accepted 14 April 2011
Keywords:
Preterm labour Tocolytic
Economic evaluation Atosiban Betamimetics
ABSTRACT
The aim of this study was to determine the cost effectiveness of atosiban compared to betamimetics in the treatment of preterm labour within the Italian setting. A systematic literature review identified randomised controlled trials (RCTs) comparing atosiban with betamimetics. Meta-analysis of nine RCTs determined that atosiban and betamimetics had similar efficacy in delaying preterm birth by at least 48 h (p = 0.910). Use of atosiban was associated with significantly fewer adverse events (p < 0.008). Results demonstrate that atosiban is cost-saving versus ritodrine or isoxuprine. Atosiban cost savings are s657 per patient from the National Health Service payer’s perspective; s299 at 18 h of tocolysis to s189 at 48 h from the hospital’s perspective. The respective values versus isoxuprine were s303 and s199. From the combined perspective, using atosiban versus ritodrine saved from s425 to s316; and versus isoxuprine from s429 to s326. Owing to its superior safety profile, atosiban is cost-saving versus betamimetics in the treatment of preterm labour in Italy from the payer’s, hospital’s and combined perspectives. With the approximate 40,000 annual preterm births in Italy the annual savings could be in excess of s13 million for the payer or s3.8–6.2 million for the hospitals.
ß 2011 Elsevier Ireland Ltd. All rights reserved.
| 1 | Introduction |
| 2 | Materials and methods |
| 3 | Results |
| 4 | Discussion |
| 5 | Conclusions |
| 6 | Acknowledgements |
| 7 | References |
1. Introduction
Preterm birth (PTB) complicates 5–12% of all pregnancies [1,2], varying from 6.2% in Europe to 11.9% in Africa [3]. Of all preterm births, 40–50% are associated with preterm labour [4,5]. In Italy, PTB has been reported in 6.5% (with 0.85% at <32 weeks) [6] or 7.2% of total births and 6.8% of live-births [7,8]. Over recent decades, the frequency of preterm birth in most Western countries appears to have been increasing [9,10]. The increased incidence is
* Corresponding author. Tel.: +44 02030 511 424; fax: +44 02030 511 435. E-mail address: jaro.wex@pharmarchitecture.com (J. Wex).
0301-2115/$ – see front matter ß 2011 Elsevier Ireland Ltd. All rights reserved. doi:10.1016/j.ejogrb.2011.04.009
multifaceted and may be related, at least in part, to the lowering of the gestational age at which the neonate can survive (i.e. 22 weeks of gestation), increased use of ultrasonography to establish gestational age [11], and changes in the definitions of fetal loss, stillbirth and early neonatal death [12,13].
A large proportion of PTB is preventable. The ultimate goal of delaying preterm birth is to allow gestation to continue until fetal maturity is achieved (i.e. >36 weeks), but an accepted surrogate outcome is to prolong pregnancy (e.g. by at least 48 h) until the mother can be transferred to a tertiary centre equipped for high- risk pregnancies with a dedicated neonatal unit. Delay of preterm labour allows for administration of corticosteroids, which accel- erates fetal lung maturation and reduces the risk of neonatal death,
J. Wex et al. / European Journal of Obstetrics & Gynecology and Reproductive Biology 157 (2011) 128–135 129
respiratory distress syndrome, cerebro-ventricular hemorrhage, infectious morbidity, necrotising enterocolitis, use of respiratory support and admission to the neonatal intensive care unit [14]. Between 22 and 28 week each day of delay improves survival by 3% [15].
The rationale for using tocolytics is that prolonging pregnancy will reduce perinatal morbidity and mortality. All licensed tocolytics are currently considered equally effective [16]. The focus is therefore on differences in maternal and fetal safety, cost of treatment, cost consequences of treatment and quality of life (QoL). There are no studies of tocolytics to date of sufficient sample size to be able to demonstrate a benefit in mortality and/or morbidity [17–19]. This only emphasizes the importance of safety outcomes, which have been established with statistical signifi- cance, especially nearer term, when neonatal mortality is less likely to be affected by treatment, yet it is when the majority of preterm labour presentations occur.
The main classes of tocolytics are betamimetics, calcium-channel blockers and vasopressin/oxytocin receptor antagonists, with atosiban and nifedipine being the main tocolytic agents currently used in clinical practice [5]. Betamimetics (e.g. ritodrine, isoxuprine) have been widely used for 30 years, but are being gradually phased out since the early 1990s due to maternal and fetal safety concerns [20]. Occurrence of side-effects is associated with the mechanism of action of betamimetics, affecting multiple functions via ubiquitous beta-adrenergic receptors. Both ritodrine and isoxuprine are licensed in Italy, but like all betamimetics, high frequency of unpleasant and sometimes severe side-effects may limit their desirability as a first-line agent [21]. Furthermore, in Italy betamimetics are contraindicated or should be used with caution in hyperthyroidism, cardiovascular diseases, arrhythmias with long QT, hypertension, and diabetes, and are contraindicated in multiple pregnancy due increased risk of pulmonary oedema [22–24].
With similar efficacy, the clinical advantage of atosiban results from its superior safety profile, with a significantly lower rate of fetal and maternal side-effects and a significantly lower rate of treatment discontinuation [25]. In a recent study from the Netherlands and Belgium, use of betamimetics was associated with higher incidence of mild and severe side-effects compared with atosiban (R.R = 24.5 [3, 197.5]) [26]. Also nifedipine had an inferior safety profile (R.R = 13.5 [1.7, 102.8]) and safety concerns for the use of calcium channel blockers in pregnancy have been raised [27]. In addition, the incidence of side-effects increased when combined courses of tocolytic agents were used. The study concluded that betamimetics should no longer be used, combined courses of tocolytic agents should not be administered and use of atosiban should be considered especially in cases of multiple gestation, diabetes and maternal cardiovascular problems [26]. Additionally, the quality of nifedipine studies has been brought into question [28]. Taking into account safety of tocolytic treatment, the UK’s Royal College of Obstetricians and Gynaecol- ogists (RCOG) recommends that oxytocin receptor antagonists (e.g. atosiban) be used as one of the first lines of treatment [29].
In Italy, atosiban is considered for acute tocolysis as the first-line agent for its efficacy and safety, though cost of treatment is indicated as its ‘‘principal side-effect’’ [21]. Also nifedipine is a consideration for tocolysis [30], but in Italy it is not registered for this indication.
PTB incurs considerable inpatient cost, and use of tocolytics is central in delaying birth. Resource utilisation associated with PTB, including maternal hospital admission, in utero transfer and neonatal care, is a burden on parents and society as a whole [31]. In addition to the economic burden on health services and enormous negative psychosocial and emotional effect on the family, the morbidity and mortality associated with PTB impose an immense burden on the education system and social services [17]. Accounting for costs of healthcare, education and social services,
the incremental cost per preterm child surviving to 18 years compared with a term survivor was estimated in the UK at £22,885. The corresponding estimates for a very and extremely preterm child were substantially higher at £61,781 and £94,740, respectively [32].
The economic implications of extremely preterm birth (<28 weeks) are well known, and early PTB (28–32 weeks) is also associated with high perinatal mortality and morbidity with economic implications. A study in the UK revealed that of the £2946 billion annual economic burden of PTB to the public sector, £1956 billion (66.4%) is attributable to moderate disability PTB [32]. The overall cost burden remains high for mildly preterm birth (32–36 weeks) and then doubles for late preterm at 37–38 weeks. The greatest risk of mortality and morbidity is for those infants born at the earliest gestational ages. However, infants born nearer to term represent the greatest number among all infants born preterm and at the same time experience more complications than infants born at term [33]. The aggregate annual cost of treating PTB at <33 weeks in England and Wales was estimated to amount to £1 billion; whereas for those born at 33–36 weeks the cost was nearly double at £1.9 billion [32]. For this reason, prevention of PTB with tocolytics is just as important, if not more important, than prevention of early PTB associated with higher cost per case.
Choice of the tocolytic has considerable economic implications. It has recently been established that atosiban, despite its greater per vial cost, is actually cost-saving when compared to betamimetics in Germany [34] and Austria [35]; cost-savings resulted from the superior safety profile of atosiban. In the UK, evidence on side-effects associated with the use of nifedipine in tocolysis was incorporated in an economic evaluation [31]. The authors found that the protocol combining atosiban with fetal fibronectin diagnostic testing is highly cost-saving when compared to nifedipine alone. An economic evaluation of specific interventions that aim to prevent PTB would allow resources to be allocated in both a clinically and cost-effective manner [17]. The aim of this study was to assess the economic implications of the choice of tocolytics in the Italian setting.
2. Materials and methods
MEDLINE, EMBASE, the Centre for Reviews and Dissemination (CRD) and the Cochrane Central Register of Controlled Trials (CENTRAL) databases were systematically searched to identify randomized clinical trials comparing atosiban versus betami- metics in women experiencing preterm labour. No search restrictions were applied and all abstracts identified using the keywords ‘‘atosiban’’, ‘‘Tractocile’’, ‘‘antocin’’, and/or ‘‘RWJ 22164’’ were considered. For the meta-analysis of efficacy and rates of adverse events, we included trials that provided outcomes during 48h of hospitalisation. Three types of outcomes data were extracted from the selected studies: efficacy in delaying preterm birth by at least 48 h, frequency of maternal and fetal adverse events, and resource utilisation for the economic analysis. Three double-blinded studies were identified. In addition, six non- double-blinded (low-quality) studies were identified and included in a separate meta-analysis. The inclusion and exclusion criteria in the remaining six studies differed from the above in minor details. In one low-quality study usual care, rather than a betamimetic, was used as a comparator of atosiban [36], but since it was a large study with most patients (64.5%) receiving betamimetics, it was included in the meta-analysis of combined high and low-quality studies. In this study the remaining patients in the usual care arm received either a calcium channel blocker (14.8%) or a betamimetic with magnesium (10%), more likely leading to under-estimation, rather than over-estimation of side-effects of betamimetics. The quality characteristics of all identified trials are presented in Tables 1 and 2. Using the Mantel–Haenszel method (random-effects


model), we performed evidence synthesis using a dedicated statistical package [37].
A cost-minimisation analysis, rather than a cost-effectiveness analysis, was conducted due to the similar efficacy of the analysed tocolytic treatments. A review of resource utilisation and expert opinion revealed that costs related to drug administration were not different and could not be captured in the Italian payment system. Diagnostic costs attributable in cases when the patient was ineligible for betamimetics (e.g. high cardiovascular risk, diabetes, thyrotoxicosis) were not considered, as in such cases alternative tocolytic treatment is typically preferred in clinical practice instead of diagnostic testing. Finally, regardless of the tocolytic agent, it was assumed that the costs of monitoring of tocolysis, as well as all other costs related to management of preterm labour and preterm birth, would be the same in all women experiencing preterm labour. Drug costs were evaluated at 18 and 48 h from the time of hospital admission. For increased accuracy, extended hospitalisation for treatment of emergency adverse events occurring within the 48h period was also factored into the analysis.
The dosing regimen used for the calculation of costs of drugs was based on the protocols used in the included clinical trials [38– 40]. Atosiban was administered intravenously in a single bolus dose (6.75 mg in 0.9 mL normal saline), with subsequent infusion of 300 mcg/min atosiban in 5% dextrose for the first 3 h, followed by 100 mcg/min atosiban in 5% dextrose for up to 48-h. The drug regimen for betamimetics was concordant with current practice guidelines for the management of preterm labour.
Ritodrine: 2 vials (50 mg) of miolene in 500cc saline; the initial dose 50–100 mcg/min increasing by 50 mcg/min every 10–30 min in the absence of contraindications (thyroid disease or diabetes mellitus) and side-effects (maternal: hyperglycemia, hyperinsuli- nemia, hypokalemia, fetal tachycardia, intraventricular hemor- rhage); the maximum dose reached 350 mcg [41]. Isoxsuprine: 8 vials (10 mg) of vasosuprina in 500 mL saline; the initial dose 30 drops/min and increasing till uterine contractile activity decreases (not exceeding 60 drops/min). Continued at full dosage until the regression of the contractile activity and then reduced to the lowest effective dose [15]. The following unit costs of tocolytics, current as of January 2010, were used in the evaluation: tractocile (atosiban) (6.75 mg): s24.18; tractocile (atosiban) (37.5 mg): s75.42; miolene (ritodrine) (50 mg): s0.52; vasosuprina (iso- xsuprine) (10 mg): s0.24. length of stay had cost implications for the hospital, even if no DRG recoding was possible, and hence no additional payments were due from the payer. The costs from the combined perspective were also calculated, as they can provide additional, whole-system insight for decision making.
The cost-minimisation analyses were conducted using an economic model developed by the authors in a Microsoft Excel spreadsheet; the model accounted for the payer–provider split characteristic of the Italian system. The modelled cohort of 1000 patients was followed up for up to 48-h of hospitalisation. Tocolytic treatments were assigned based on the all-patients treated population from the combined clinical trials. Discontinua- tion of drug administration due to adverse events, progression of labour, preterm delivery and other causes was accounted for. Weibull survival curves were fitted to the data on discontinuation at 48 h, preterm delivery at 48 h, and at 7 days. Drug switching was assumed to occur with equal probability during the 48h hospitalisation period. Hospitalisation length was defined based on expert opinion using beta distribution with the mean of 2.2 days, minimum of 1 day and maximum of 10 days. Occurrence of adverse events was associated with risk of extension of the hospitalisation length. It was assumed that on average only 50% of the patients experiencing any of the adverse events would require hospitalisation extended by one or more days. Occurrence of multiple adverse events was assumed to have the same consequences as occurrence of any single event. Effectively, we conservatively assumed no consequences of occurrence of adverse events in 50% of the patients. Similarly, diagnosis of chest pain or dyspnoea could lead to recoding in 50% of the patients.
In Italy, ritodrine and isoxuprine are the only betamimetic agents indicated for preterm labour, but an analysis of adverse events reported in trials of three different betamimetics demon- strated their comparable safety profile [19]. DRG tariffs were obtained with DRG Grouper v.19 using national schedule applica- ble to most regions. From the National Health Service payer’s perspective, all costs associated with treatment of preterm labour were encompassed by the flat DRG rates per patient diagnosed. For the payer, only extended length of stay and occurrence of chest pain or dyspnoea had cost consequences resulting from DRG recoding. From the hospital’s perspective, every extension of
3. Results
Based on three high-quality studies [38–40], efficacy of atosiban in delaying preterm birth by at least 48-h was found to be identical to that of betamimetics (88.1% vs. 88.7%, respectively, p = 0.910) (OR = 0.94 [0.59, 1.48]) (Fig. 1). Addition of two single- blinded [42,43], two open label [1,44,45], and one study with usual care in the control arm [36], resulted in slightly higher success rate for atosiban and lower for betamimetics (90.1% vs. 88.6%), but also with no significant difference (p = 0.61) (OR = 1.14 [0.82, 1.56]) (Fig. 2).
Meta-analysis of the three double-blinded clinical trials revealed that use of atosiban was associated with significantly lower frequency of adverse events compared to betamimetics (for individual adverse events p-value varied from <0.001 to 0.008). Compared to betamimetics, use of atosiban was associated with a significantly lower frequency of adverse events for tachycardia (5.5% vs. 75.5%; OR = 0.02 [0.00, 0.05]), fetal tachycardia (3.3% vs. 27.7%; OR = 0.10 [0.05, 0.22]), dyspnoea (0.2% vs. 7.3%; OR = 0.07 [0.02, 0.31]), chest pain (1.1% vs. 4.8%; OR = 0.21 [0.07, 0.63]), palpitation, vomiting, headache, hyperglycaemia, tremor, hypo- calemia. Inclusion of all nine studies yielded similar safety advantage of atosiban with the frequency of tachycardia (3.6% vs. 56.4%; OR = 0.02 [0.01, 0.04]); fetal tachycardia (9.3% vs. 26.2%;
J. Wex et al. / European Journal of Obstetrics & Gynecology and Reproductive Biology 157 (2011) 128–135

Fig. 1. Undelivered rate as a measure of efficacy of tocolysis based on evidence from the high-quality clinical trials. Summary odds ratio (Mantel–Henschel) = 0.935 (95% CI: 0.593–1.475).
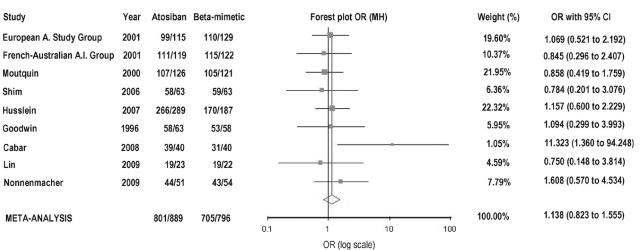
Fig. 2. Undelivered rate as a measure of efficacy of tocolysis based on evidence from all nine clinical trials. Summary odds ratio (Mantel–Haenszel) = 1.138 (95% CI: 0.823– 1.555).
OR = 0.18 [0.05, 0.56]); dyspnoea (0.6% vs. 8.5%; OR = 0.09 [0.04, 0.22]) and chest pain (1.6% vs. 8.9%; OR = 0.17 [0.08, 0.36]); with p- value <0.001 for each adverse event.
Cost results for the three treatment options based on high- quality trials are presented in Fig. 3. From the payer’s perspective, cost-saving from using atosiban versus either beta-mimetic was s657 per patient. From the hospital’s perspective, savings from using atosiban versus ritodrine ranged from s299 for 18-h of tocolysis to s189 for 48-h; the respective values for isoxuprine were s303 and s199. From the combined perspective, using atosiban versus ritodrine saved from s425 for 18-h of tocolysis to s316 for 48-h; versus isoxuprine the results were s429 and s326, respectively. When all nine RCTs were considered, cost-saving from the payer’s perspective using atosiban versus either betamimetic was s646 per patient. From the hospital’s perspec- tive, savings from using atosiban versus ritodrine ranged from s261 for 18-h of tocolysis to s152 for 48-h; the respective values for isoxuprine were s265 and s161. From the combined perspective, using atosiban versus ritodrine saved from s414 for 18-h of tocolysis to s304 for 48-h; versus isoxuprine the results were s418 and s315, respectively. The results were robust in the probabilistic sensitivity analyses (PSA), where cost-savings in all scenarios was achieved in 98.2–100% of cases. Twenty percent variation in DRG tariff to account for regional differences was factored in the PSA.
With the approximate 40,000 annual preterm births in Italy and based on the assumption that only half of women who deliver preterm are treated with tocolytics the annual savings could be in excess of s13 million for the payer or s3.8–6.2 million for the hospitals.
OR = 0.18 [0.05, 0.56]); dyspnoea (0.6% vs. 8.5%; OR = 0.09 [0.04, 0.22]) and chest pain (1.6% vs. 8.9%; OR = 0.17 [0.08, 0.36]); with p- value <0.001 for each adverse event.
Cost results for the three treatment options based on high- quality trials are presented in Fig. 3. From the payer’s perspective, cost-saving from using atosiban versus either beta-mimetic was s657 per patient. From the hospital’s perspective, savings from using atosiban versus ritodrine ranged from s299 for 18-h of tocolysis to s189 for 48-h; the respective values for isoxuprine were s303 and s199. From the combined perspective, using atosiban versus ritodrine saved from s425 for 18-h of tocolysis to s316 for 48-h; versus isoxuprine the results were s429 and s326, respectively. When all nine RCTs were considered, cost-saving from the payer’s perspective using atosiban versus either betamimetic was s646 per patient. From the hospital’s perspec- tive, savings from using atosiban versus ritodrine ranged from s261 for 18-h of tocolysis to s152 for 48-h; the respective values for isoxuprine were s265 and s161. From the combined perspective, using atosiban versus ritodrine saved from s414 for 18-h of tocolysis to s304 for 48-h; versus isoxuprine the results were s418 and s315, respectively. The results were robust in the probabilistic sensitivity analyses (PSA), where cost-savings in all scenarios was achieved in 98.2–100% of cases. Twenty percent variation in DRG tariff to account for regional differences was factored in the PSA.
With the approximate 40,000 annual preterm births in Italy and based on the assumption that only half of women who deliver preterm are treated with tocolytics the annual savings could be in excess of s13 million for the payer or s3.8–6.2 million for the hospitals.
4. Discussion
The economic evaluation comparing atosiban to betamimetics in Italy demonstrated that considerable cost-savings can be achieved with atosiban due to reduction in costs of treatment associated with side-effects, which were significantly more frequent with betamimetics.
Our analysis was based on efficacy data from a meta-analysis of clinical trials. It is has been suggested, however, that any meta- analysis that only includes RCTs is likely to significantly under- estimate adverse events and safety concerns due to the focus of the studies on efficacy as primary endpoints [46–48]. Clinical trials typically enrol low-risk patients without comorbidities, which may potentiate harmful side-effects. Indeed, most meta-analyses only include RCTs because they are mainly performed for efficacy data. With respect to safety, RCTs alone do not provide a sufficient evidence base [27,49]. In an Italian observational study, 30% of patients treated with isoxuprine stopped treatment due to occurrence of maternal and fetal side-effects (0% in the atosiban treated patients) [50]. In this study the following side-effects were reported for isoxuprine vs. atosiban: nausea and vomiting (20% vs. 20%), maternal tachycardia (60% vs. 0%), fetal tachycardia (50% vs. 0%), headache (20% vs. 10%), tremor (10% vs. 0%), hypotension (10% vs. 0%), palpitations (10% vs. 0%). In another study ritodrine was compared to atosiban in patients with preterm birth following ICSI. Tachycardia was reported in 15/16 patients on ritodrine and 1/16 patients on atosiban [51].
Previously conducted economic evaluations of atosiban versus betamimetics demonstrated conflicting results. In a German setting, atosiban was shown to be cost-savings versus betami-
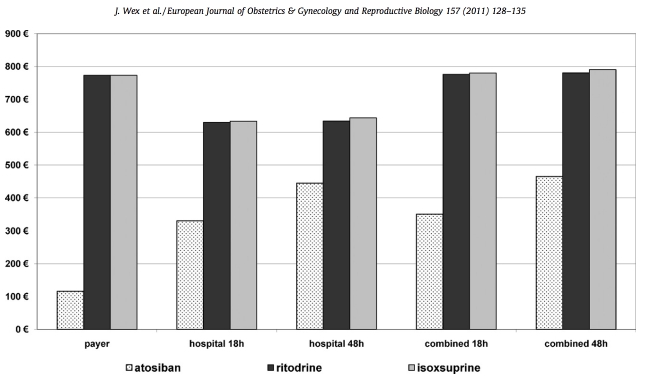
Fig. 3. Cost results for the three tocolytic treatment options and the three perspectives with different time horizons, based on evidence from the high-quality clinical trials.
metics from the payer, hospital and combined perspectives [34]. In a Spanish economic study the average cost per patient treated with atosiban was greater than with ritodrine, and atosiban was judged more expensive and less effective [52], but the critical assumption of higher efficacy of ritodrine was not grounded in statistical significance and was not supported by recent evaluations. For the same reason of lack of statistical significance, inclusion of pulmonary oedema among the adverse events was not warranted, while other adverse events were not considered in the Spanish analysis.
In our analysis, we have made assumptions and simplifications which we deemed conservative and likely to under-estimate the superiority of atosiban. For example, immediate treatment scenario with immediate treatment reported to lead to higher success rate [53] was not considered. A clinical trial carried out at 105 centres in six European countries established that atosiban given without delay was more effective compared with adminis- tration of atosiban at the standard time: a significantly greater proportion of women remained undelivered without the need for an alternative tocolytic (88.9% vs. 76.1%, p = 0.03) [53]. Incorpo- rating efficacy data from this study would produce even more cost- savings for early initiation of treatment with atosiban compared with betamimetics. Also the disutility resulting from side-effects was not included in the model due to lack of accurate data, although a QoL scale has been previously used for comparing betamimetics [54]. We also did not account for patient satisfaction, although 59.6% of patients treated with atosiban had been reported to be satisfied at discharge, compared to 27% with usual care [36].
In our analysis we included only side-effects for which the difference between treatments was statistically significant, which might have led to an under-estimate. Based on the three high- quality studies, there was a trend for higher risk of caesarean section in the betamimetics group than in the atosiban group. Caesarean section constitutes a considerable cost item with both clinical and further cost consequences. In accordance with clinical trial protocols, we did not capture cost consequences of switching from beta-mimetic to atosiban, which was not allowed in the trials and effectively led to under-estimation of the costs in the patients randomised to betamimetics. The analysis was conservative in that only direct medical costs associated with adverse events, excluding non-medical costs and costs of lost productivity (e.g. due to longer hospital stay) were considered. Furthermore, the diagnostic costs associated with safety concerns and contraindications were not considered, most of them being captured by the DRG schedule. Finally, the longer-term inefficiencies due to crudeness of DRG tariffs not accounting for side-effects were not analysed, but the logic of the DRG system is predicated on updated analyses allowing for additional cost items to be incorporated in the tariff to reflect real costs for the provider and to maintain incentive for cost- effective health care.
5. Conclusions
Atosiban is cost-saving versus betamimetics in the treatment of preterm labour in Italy from the payer’s, hospital’s and combined perspectives. These cost-savings resulted from the superior safety profile of atosiban. The results were robust in the probabilistic sensitivity analysis. With the approximate 40,000 annual preterm births in Italy, and based on the assumption that half the women who deliver preterm are treated with tocolytics, the annual savings could be in excess of s13 million for the payer or s3.8–6.2 million for the hospitals.
Acknowledgements
JW is a director of an independent consultancy, who received an unrestricted research grant from Ferring Pharmaceuticals to study cost-effectiveness of tocolytic treatments. The sponsor was not involved in the study. AS was involved in the study through the consultancy. GC and GCDR were not remunerated for their contribution and declared no competing interests. JW conceived of the analytical approach, contributed to the literature review, designed the economic model and drafted the manuscript. AS contributed to the analysis of data and to the review clinical evidence. GC and GCDR contributed to the analysis and interpre- tation of data and provided clinical information for costing study. All authors revised the manuscript and have given their final approval for submission.
References
[1] Nonnenmacher A, Hopp H, Dudenhausen J. Effectiveness and safety of atosiban vs. pulsatile administration of fenoterol in the treatment of preterm labour. Z Geburtshilfe Neonatol 2009;213(October (5)):201–6.
[2] Slattery MM, Morrison JJ. Preterm delivery. Lancet 2002;360(November (9344)):1489–97.
[3] Beck S, Wojdyla D, Say L, et al. WHO systematic review on maternal mortality and morbidity: the global burden of preterm birth. Bull World Health Organ 2009.
[4] Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: final data for 2004. Natl Vital Stat Rep 2006;55(September (1)):1–101.
[5] Sanu O, Lamont RF. Critical appraisal and clinical utility of atosiban in the management of preterm labor. Ther Clin Risk Manage 2010;6:191–9.
[6] Salute Md. Certificato di assistenza al parto (CeDAP). Analisi dell’evento nascita–Anno 2005. Quarta edizione; April 2008.
[7] Gissler M, Mohangoo AD, Blondel B, et al. Perinatal health monitoring in Europe: results from the EURO-PERISTAT project. Inform Health Soc Care 2010;35(March (2)):64–79.
[8] Zimbeck M, Mohangoo A, Zeitlin J. The European perinatal health report: delivering comparable data for examining differences in maternal and infant health. Eur J Obstet Gynecol Reprod Biol 2009;146(October (2)):149–51.
[9] de Heus R, Mulder E, Visser GH. Management of preterm labor: atosiban or nifedipine? Int J Women Health 2010;2:137–42.
[10] Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics 2006;117(January (1)):168–83.
[11] Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 2010;88(January (1)):31–8.
[12] Davidoff MJ, Dias T, Damus K, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol 2006;30(February (1)):8–15.
[13] Stanton C, Lawn JE, Rahman H, Wilczynska-Ketende K, Hill K. Stillbirth rates: delivering estimates in 190 countries. Lancet 2006;367(May (9521)):1487–94.
[14] Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev
2006;3:CD004454.
[15] Di Renzo GC, Roura LC. Guidelines for the management of spontaneous
preterm labor. J Perinat Med 2006;34(5):359–66 [Review].
[16] Papatsonis D, Flenady V, Cole S, Liley H. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst Rev 2005;(3):CD004452.
[17] Petraglia F, Visser GH. Prevention and management of preterm labour. J
Matern Fetal Neonatal Med 2009;22(Suppl. 2):24–30.
[18] Lyndrup J, Lamont RF. The choice of a tocolytic for the treatment of preterm
labor: a critical evaluation of nifedipine versus atosiban. Expert Opin Investig
Drugs 2007;16(June (6)):843–53.
[19] Treatment of preterm labor with the beta-adrenergic agonist ritodrine. The
Canadian Preterm Labor Investigators Group. N Engl J Med. 1992;327 July
(5):308–312.
[20] Lamont R, van Eyck J. Tocolysis with nifedipine or beta-adrenergic agonists: a
meta-analysis. Obstet Gynecol 2002;99(March (3)):518–9. author reply 9–20.
[21] Maloni JA, Damato EG. Reducing the risk for preterm birth: evidence and implications for neonatal nurses. Adv Neonatal Care 2004;4(June (3)):166–74.
[22] Panella M, Garozzo V, Librino A. Tocolisi. Moderni orientamenti. 828 Congresso
Nazionale SIGO; 2006.
[23] Del Savio F, Cornacchia L, Civitella C, et al. Minaccia di parto prematuro:
Razionale di trattamento. 838 Congresso Nazionale SIGO; 2007.
[24] Gazzetta Ufficiale della Repubblica Italiana. Modifica degli stampati e del regime di fornitura di specialita‘medicinali contenenti ritodrina e isoxisuprina.
Serie Generale n 166 del 19/7/2003; 2003.
[25] Chan J, Cabrol D, Ingemarsson I, Marsal K, Moutquin J-M, Fisk NM. Pragmatic
comparison of beta2-agonist side effects within the Worldwide Atosiban versus Beta Agonists study. Eur J Obstet Gynecol Reprod Biol. [Comparative Study Multicenter Study Randomized Controlled Trial Research Support, Non- U.S. Gov’t]. 2006;128 (September–October (1–2)):135–141.
[26] de Heus R, Mol BW, Erwich JJ, et al. Adverse drug reactions to tocolytic treatment for preterm labour: prospective cohort study. BMJ 2009;338:b744.
[27] Khan K, Zamora J, Lamont RF, et al. Safety concerns for the use of calcium channel blockers in pregnancy for the treatment of spontaneous preterm labour and hypertension: a systematic review and meta-regression analysis. J
Matern Fetal Neonatal Med 2010;23(September (9)):1030–8.
[28] Lamont RF, Khan KS, Beattie B, et al. The quality of nifedipine studies used to assess tocolytic efficacy: a systematic review. J Perinat Med 2005;33(4):287–95.
[29] Rcog. Tocolytic drugs for women in preterm labour: Royal College of Obste-
tricians and Gynaecologists (RCOG). 2002.
[30] Conoscenti G, Calı` G, Zarbo R, Scollo P. Parto pretermine: presentazione delle linee guida. 858 Congresso Nazionale SIGO; 2009.
[31] SiassakosD,O’BrienK,DraycottT.Healthcareevaluationoftheuseofatosiban and fibronectin for the management of pre-term labour. J Obstet Gynaecol 2009;29(August (6)):507–11.
[32] Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics 2009;123(Feb- ruary (2)):e312–27.
[33] Institute of Medicine (US) Committee on understanding premature birth, assuring healthy outcomes. Behrman REB, editor. Preterm birth: causes, consequences, and prevention. Washington, DC: National Academies Press (US); 2007.
[34] Wex J, Connolly M, Rath W. Atosiban versus betamimetics in the treatment of preterm labour in Germany: an economic evaluation. BMC Pregnancy Child- birth 2009;9:23.
[35] Wex J, Helmer H, Rath W, Nielsen SK. Economic evaluation of atosiban compared to betamimetics for the treatment of the treatment of preterm labour in Austria. Value Health 2009;12(7):A294.
[36] Husslein P, Cabero Roura L, Dudenhausen JW, et al. Atosiban versus usual care for the management of preterm labor. J Perinat Med 2007;35(4): 305–13.
[37] Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol 2006;6:50.
[38] European Atosiban Study Group. The oxytocin antagonist atosiban versus the B-agonist Terbutaline in the treatment of preterm labour. A randomised, double-blind, controlled study. Acta Obstet Gynecol Scand 2001;80(May (5)):413–22.
[39] Moutquin JM, Sherman D, Cohen H, et al. Double-blind, randomized, con- trolled trial of atosiban and ritodrine in the treatment of preterm labor: a multicenter effectiveness and safety study. Am J Obstet Gynecol 2000;182(May (5)):1191–9.
[40] French/Australian Atosiban Investigators Group. Treatment of preterm labor with the oxytocin antagonist atosiban: a double-blind, randomized, controlled comparison with salbutamol. Eur J Obstet Gynecol Reprod Biol 2001;98(Feb- ruary (2)):177–85.
[41] Corosu R, Tillo R, Franceschetti S. Management del parto pretermine. 2010 [24 Novemeber 2010]; Available from: http://www.sippo.it/PDF/management _del_parto_pretermine.pdf.
[42] Goodwin TM, Valenzuela GJ, Silver H, Creasy G, Atosiban Study Group. Dose ranging study of the oxytocin antagonist atosiban in the treatment of preterm labor. Obstet Gynecol 1996;88(September (3)):331–6.
[43] Shim JY, Park YW, Yoon BH, et al. Multicentre, parallel group, randomised, single-blind study of the safety and efficacy of atosiban versus ritodrine in the treatment of acute preterm labour in Korean women. BJOG 2006;113(Novem- ber (11)):1228–34.
[44] Cabar FR, Bittar RE, Gomes CM, Zugaib M. Atosiban as a tocolytic agent: a new proposal of a therapeutic approach. Rev Bras Gynecol Obstet 2008;February (30)(2):87–92.
[45] Lin CH, Lin SY, Shyu MK, Chen SU, Lee CN. Randomized trial of oxytocin antagonist atosiban versus beta-adrenergic agonists in the treatment of spontaneous preterm labor in Taiwanese women. J Formos Med Assoc 2009;108(June (6)):493–501.
[46] King JF, Flenady V, Papatsonis D, Dekker G, Carbonne B. Calcium channel blockers for inhibiting preterm labour; a systematic review of the evidence and a protocol for administration of nifedipine. Aust N Z J Obstet Gynaecol 2003;43(June (3)):192–8.
[47] Oei SG, Mol BW, de Kleine MJ, Brolmann HA. Nifedipine versus ritodrine for suppression of preterm labor; a meta-analysis. Acta Obstet Gynecol Scand 1999;78(October (9)):783–8.
[48] Tsatsaris V, Papatsonis D, Goffinet F, Dekker G, Carbonne B. Tocolysis with nifedipine or beta-adrenergic agonists: a meta-analysis. Obstet Gynecol 2001;97(May (5) Pt. 2):840–7.
[49] Loke YK, Derry S. Reporting of adverse drug reactions in randomised controlled trials – a systematic survey. BMC Clin Pharmacol 2001;1:3.
[50] Merenda A, Stile A, Nazzaro G, De Placido G, D’Errico L, Locci M. Isossisprina cloridrato vs atosiban in pazienti affette da PROM. Nostra esperienza Atti dell’808 Congresso SIGO; 2006.
[51] Locci M, Nazzaro G, Merenda A, Pisaturo ML, Laviscio P, Poppiti R, et al. Atosiban vs ritodrine used prophylactically with cerclage in ICSI pregnancies to prevent pre-term birth in women identified as being at high risk on the basis of transvaginal ultrasound scan. J Obstet Gynaecol 2006;26(July (5)):396–401.
[52] Ferriols Lisart R, Nicolas Pico J, Alos Alminana M. Pharmacoeconomic assess- ment of two tocolysis protocols for the inhibition of premature delivery. Farm Hosp 2005;29(January–February (1)):18–25.
[53] Husslein P, Roura LC, Dudenhausen J, et al. Clinical practice evaluation of atosiban in preterm labour management in six European countries. BJOG 2006;113(December (Suppl. 3)):105–10.
[54] Jakovljevic M, Varjacic M, Jankovic SM. Cost-effectiveness of ritodrine and fenoterol for treatment of preterm labor in a low-middle-income country: a case study. Value Health 2008;11(March–April (2)):149–53.
